Label: CROMOLYN SODIUM solution/ drops
- NDC Code(s): 71205-230-10
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 61314-237
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
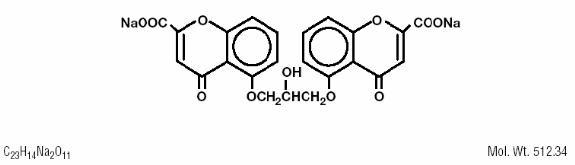
DESCRIPTION Cromolyn Sodium Ophthalmic Solution USP, 4% is a clear, colorless, sterile solution intended for topical ophthalmic use. Cromolyn sodium is represented by the following structural ...
-
CLINICAL PHARMACOLOGY In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the degranulation of sensitized mast cells which occurs after exposure to specific antigens. Cromolyn sodium acts by ...
-
INDICATIONS AND USAGE Cromolyn Sodium Ophthalmic Solution USP, 4% is indicated in the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
-
CONTRAINDICATIONS Cromolyn Sodium Ophthalmic Solution USP, 4% is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium or to any of the other ingredients.
-
PRECAUTIONS General - Patients may experience a transient stinging or burning sensation following application of Cromolyn Sodium Ophthalmic Solution USP, 4%. The recommended frequency of administration ...
-
ADVERSE REACTIONS The most frequently reported adverse reaction attributed to the use of cromolyn sodium ophthalmic solution, on the basis of reoccurrence following readministration, is transient ocular stinging or ...
-
DOSAGE AND ADMINISTRATION The dose is 1 or 2 drops in each eye 4 to 6 times a day at regular intervals. One drop contains approximately 1.6 mg cromolyn sodium. Patients should be advised that the effect of cromolyn sodium ...
-
HOW SUPPLIED Cromolyn Sodium Ophthalmic Solution USP, 4% is supplied in a white, opaque, plastic ophthalmic dispenser in the following sizes: 10 mL NDC 71205-230-10 - Storage: Store between 15° - 30°C (59° ...
-
SPL UNCLASSIFIED SECTION9007073-0811 - Manufactured by - Alcon Laboratories, Inc. Fort Worth, Texas 76134 for - Sandoz Inc. Princeton, NJ 08540 - Printed in USA - August 2011 - Relabeled by - Proficient Rx LP - Thousand Oaks, CA ...
-
PRINCIPAL DISPLAY PANEL NDC 71205-230-10 Cromolyn - Sodium - Ophthalmic - Solution USP - 4% Rx Only - STERILE - 10 mL
-
INGREDIENTS AND APPEARANCEProduct Information