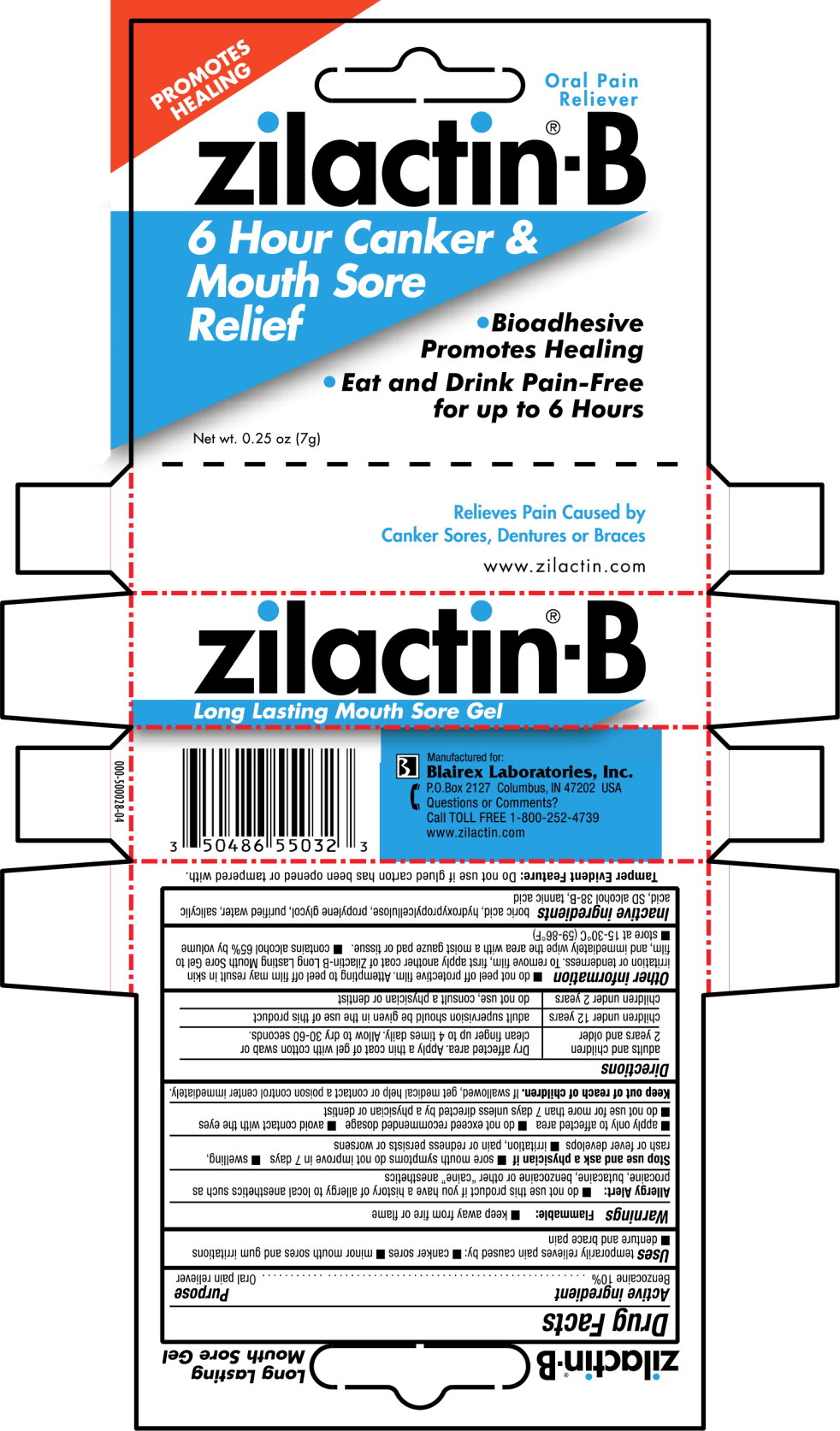
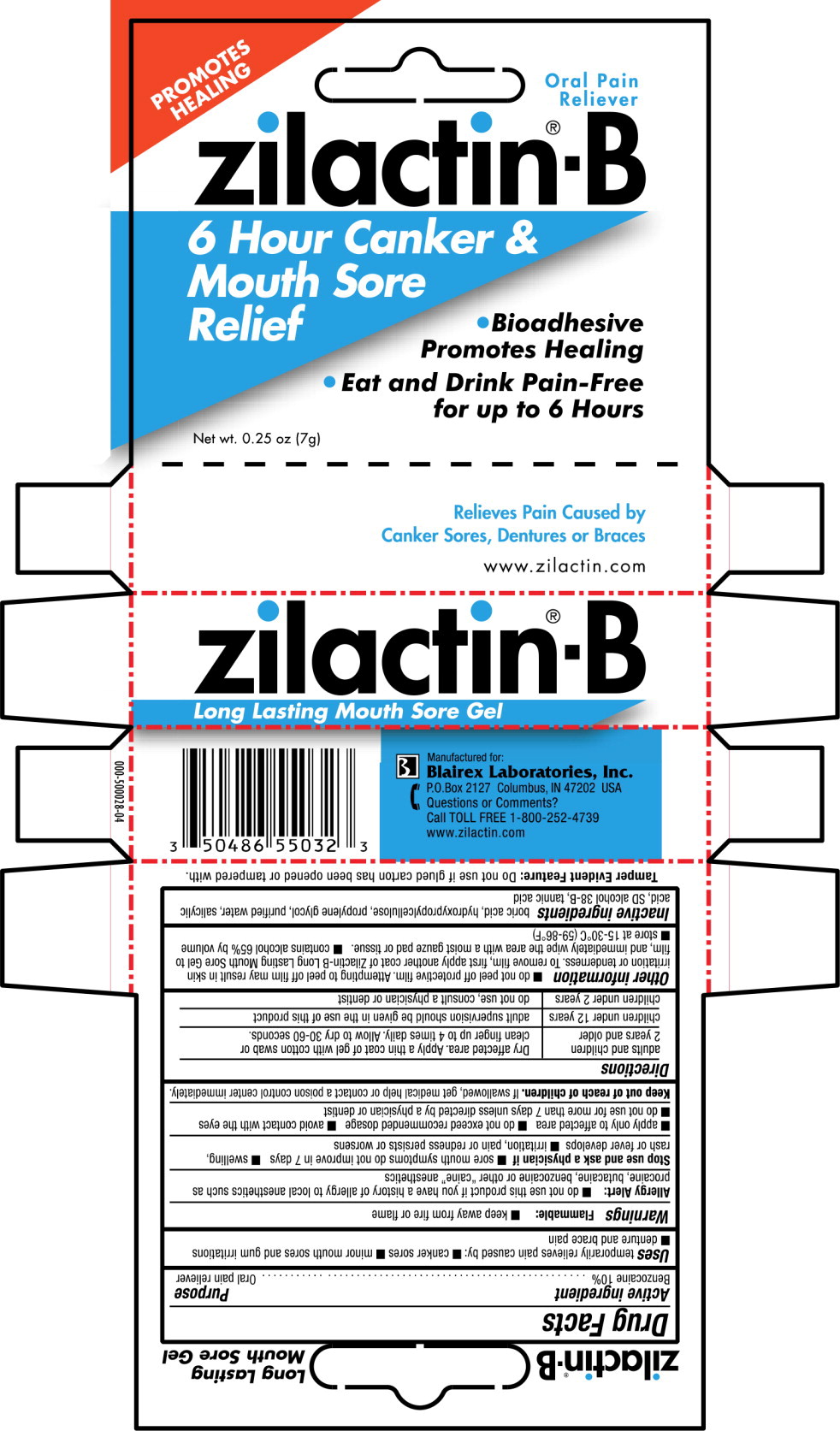
Label: ZILACTIN-B- benzocaine gel
- NDC Code(s): 50486-550-32
- Packager: Blairex Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Allergy Alert:
- do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
Stop use and ask a physician if
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
- apply only to affected area
- do not exceed recommended dosage
- avoid contact with the eyes
- do not use for more than 7 days unless directed by a physician or dentist
-
Directions
adults and children
2 years and olderDry affected area. Apply a thin coat of gel with cotton swab or clean finger up to 4 times daily. Allow to dry 30-60 seconds. children under 12 years adult supervision should be given in the use of this product children under 2 years do not use, consult a physician or dentist -
Other information
- do not peel off protective film. Attempting to peel off film may result in skin irritation or tenderness. To remove film, first apply another coat of Zilactin-B Long Lasting Mouth Sore Gel to film, and immediately wipe the area with a moist gauze pad or tissue.
- contains alcohol 65% by volume
- store at 15-30°C (59-86°F)
- Inactive ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZILACTIN-B
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50486-550 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) HYDROXYPROPYL CELLULOSE (TYPE M) (UNII: U3JF91U133) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SALICYLIC ACID (UNII: O414PZ4LPZ) ALCOHOL (UNII: 3K9958V90M) PEPPERMINT OIL (UNII: AV092KU4JH) TANNIC ACID (UNII: 28F9E0DJY6) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50486-550-32 1 in 1 CARTON 06/29/2005 1 7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 06/29/2005 Labeler - Blairex Laboratories, Inc. (092575133)

 Questions or Comments?
Questions or Comments?