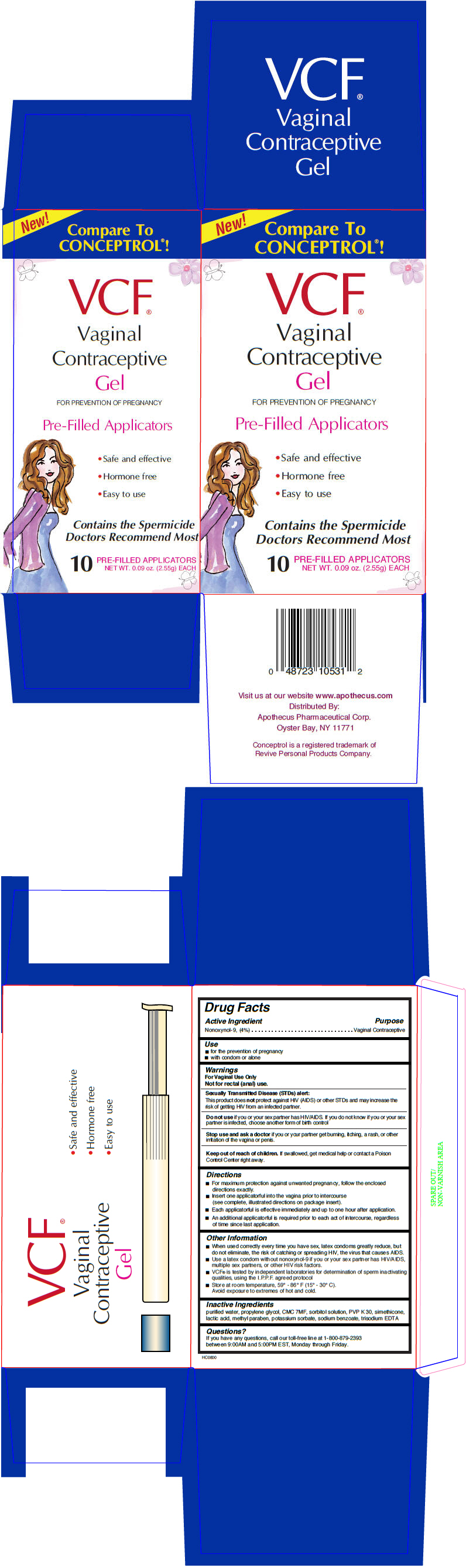
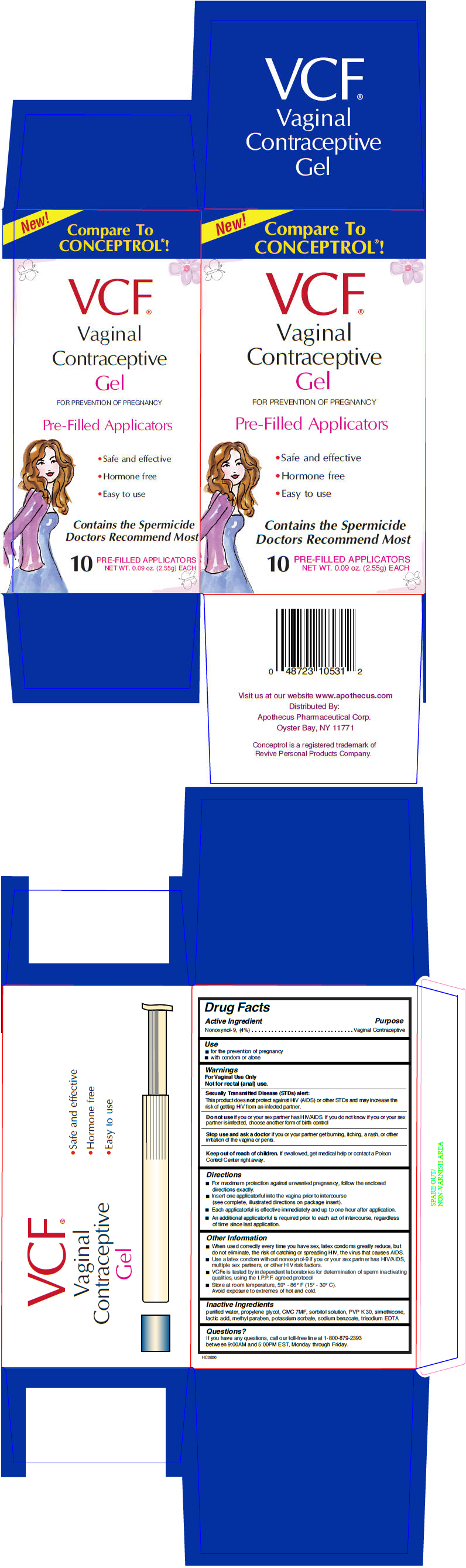
Label: VCF CONTRACEPTIVE PRE-FILLED APPLICATORS- nonoxynol-9 gel, metered
- NDC Code(s): 52925-512-10, 52925-512-25
- Packager: APOTHECUS PHARMACEUTICAL CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For Vaginal Use Only
Not for rectal (anal) use.
Sexually Transmitted Disease (STDs) alert
This product does not protect against HIV (AIDS) or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is infected, choose another form of birth control
-
Directions
- For maximum protection against unwanted pregnancy, follow the enclosed directions exactly.
- Insert one applicatorful into the vagina prior to intercourse (see complete, illustrated directions on package insert).
- Each applicatorful is effective immediately and up to one hour after application.
- An additional applicatorful is required prior to each act of intercourse, regardless of time since last application.
-
Other Information
- When used correctly every time you have sex, latex condoms greatly reduce, but do not eliminate, the risk of catching or spreading HIV, the virus that causes AIDS.
- Use a latex condom without nonoxynol-9 if you or your sex partner has HIV/AIDS, multiple sex partners, or other HIV risk factors.
- VCF® is tested by independent laboratories for determination of sperm inactivating qualities, using the I.P.P.F. agreed protocol
- Store at room temperature, 59° - 86° F (15° - 30° C).
Avoid exposure to extremes of hot and cold.
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2.55 g Applicator Box
-
INGREDIENTS AND APPEARANCE
VCF CONTRACEPTIVE PRE-FILLED APPLICATORS
nonoxynol-9 gel, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52925-512 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NONOXYNOL-9 (UNII: 48Q180SH9T) (NONOXYNOL-9 - UNII:48Q180SH9T) NONOXYNOL-9 4 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Benzoate (UNII: OJ245FE5EU) Sorbitol (UNII: 506T60A25R) Povidone K30 (UNII: U725QWY32X) Lactic Acid, Unspecified Form (UNII: 33X04XA5AT) Methylparaben (UNII: A2I8C7HI9T) Potassium Sorbate (UNII: 1VPU26JZZ4) Edetate Trisodium (UNII: 420IP921MB) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52925-512-10 10 in 1 BOX 06/01/2014 1 2.55 g in 1 APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:52925-512-25 250 in 1 CARTON 06/01/2014 2 2.55 g in 1 APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug 505G(a)(3) 06/01/2014 Labeler - APOTHECUS PHARMACEUTICAL CORP (119263747)