Label: NAFCILLIN SODIUM injection, powder, for solution
- NDC Code(s): 82449-503-02, 82449-504-02
- Packager: Steriscience Specialties Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nafcillin for Injection and other antibacterial drugs, Nafcillin for Injection should be used only ...
-
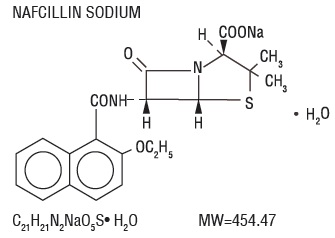
DESCRIPTIONNafcillin for Injection, USP is semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillanic acid. The chemical name of nafcillin sodium is Monosodium (2 - S,5 - R,6 ...
-
CLINICAL PHARMACOLOGYIn a study of five healthy adults administered a single 500 mg dose of nafcillin by intravenous injection over seven minutes, the mean plasma concentration of the drug was approximately 30 mcg/mL ...
-
INDICATIONS AND USAGENafcillin is indicated in the treatment of infections caused by penicillinase-producing staphylococci which have demonstrated susceptibility to the drug. Culture and susceptibility tests should be ...
-
CONTRAINDICATIONSA history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
-
WARNINGSSerious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a ...
-
PRECAUTIONSGeneral - Nafcillin should generally not be administered to patients with a history of sensitivity to any penicillin. Penicillin should be used with caution in individuals with histories of ...
-
ADVERSE REACTIONSBody as a Whole - The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent (see - WARNINGS). Sensitization is usually the result of treatment, but some ...
-
DOSAGE AND ADMINISTRATIONNafcillin for Injection is available for intramuscular and intravenous use. The usual I.V dosage for adults is 500 mg every 4 hours. For severe infections, 1 gram every 4 hours is recommended ...
-
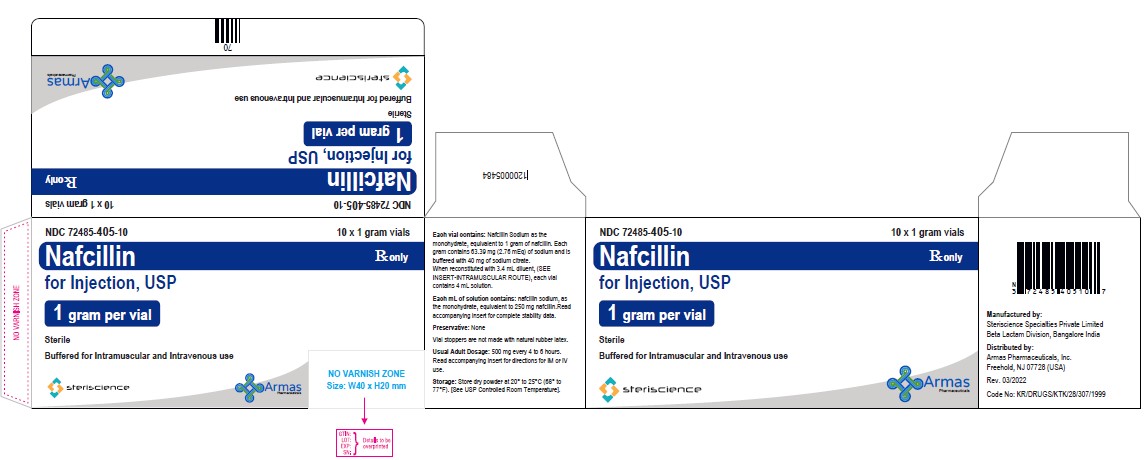
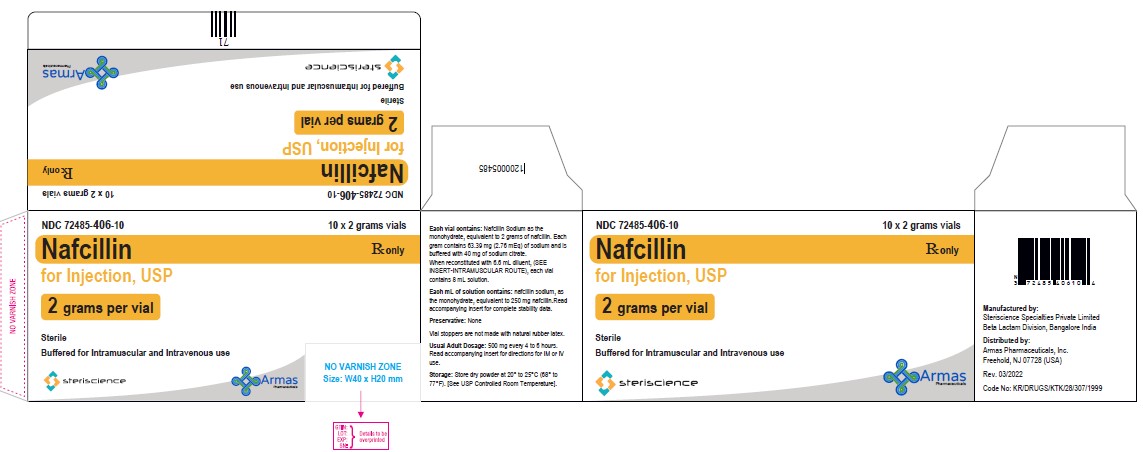
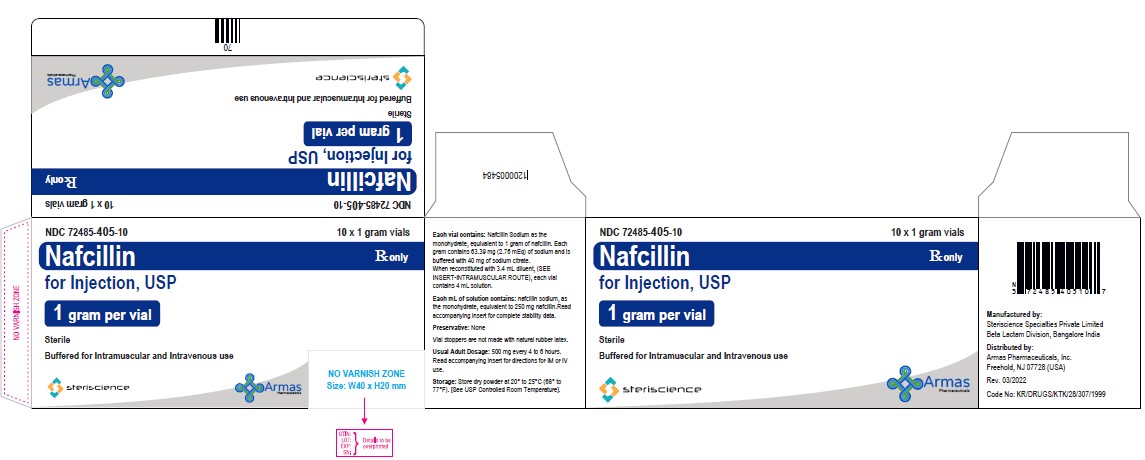
HOW SUPPLIEDEach Nafcillin for Injection, USP vial contains nafcillin sodium equivalent to 1 gram or 2 grams of nafcillin. * Vial stoppers are not made with natural rubber latex. Store dry powder at ...
-
SPL UNCLASSIFIED SECTIONManufactured by: STERISCIENCE SPECIALTIES PRIVATE LIMITED - Beta Lactam Division, Bangalore India - March 2022
-
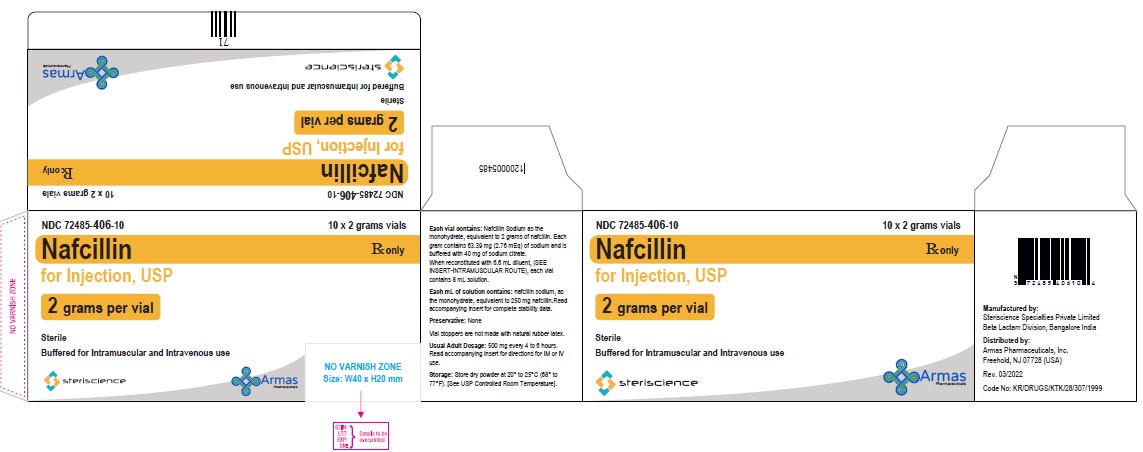
Package/Label Display PanelVial Label: NDC 82449-503-01 - Rx only - Nafcillin for Injection, USP - (1 gram per vial) Sterile - Buffered for Intramuscular and Intravenous use - Steriscience - Carton Label: NDC ...
-
PRINCIPAL DISPLAY PANELVial Label: NDC 82449-503-01 - Rx only - Nafcillin for Injection, USP - (2 grams per vial) Sterile - Buffered for Intramuscular and Intravenous use - Steriscience - Carton Label: NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information