Label: EMGALITY- galcanezumab-gnlm injection, solution
-
NDC Code(s):
0002-1436-01,
0002-1436-11,
0002-1436-27,
0002-1436-61, view more0002-2377-01, 0002-2377-11, 0002-2377-27, 0002-3115-01, 0002-3115-09
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EMGALITY safely and effectively. See full prescribing information for EMGALITY. EMGALITY (galcanezumab-gnlm) injection, for ...These highlights do not include all the information needed to use EMGALITY safely and effectively. See full prescribing information for EMGALITY.
EMGALITY (galcanezumab-gnlm) injection, for subcutaneous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For subcutaneous use only. (2.1, 2.2, 2.3)
- Migraine recommended dosage: 240 mg loading dose (administered as two consecutive injections of 120 mg each), followed by monthly doses of 120 mg. (2.1)
- Episodic cluster headache recommended dosage: 300 mg (administered as three consecutive injections of 100 mg each) at the onset of the cluster period, and then monthly until the end of the cluster period. (2.2)
- Administer in the abdomen, thigh, back of the upper arm, or buttocks subcutaneously. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
EMGALITY is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: If a serious hypersensitivity reaction occurs, discontinue administration of EMGALITY and initiate appropriate therapy. Hypersensitivity reactions can occur days after administration, and may be prolonged. (5.1)
- Hypertension: New-onset or worsening of pre-existing hypertension may occur. (5.2)
- Raynaud's Phenomenon: New-onset or worsening of pre-existing Raynaud's phenomenon may occur. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2% and at least 2% greater than placebo) in EMGALITY clinical studies were injection site reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Migraine
1.2 Episodic Cluster Headache
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing for Migraine
2.2 Recommended Dosing for Episodic Cluster Headache
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Hypertension
5.3 Raynaud's Phenomenon
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Migraine
14.2 Episodic Cluster Headache
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Migraine - EMGALITY is indicated for the preventive treatment of migraine in adults. 1.2 Episodic Cluster Headache - EMGALITY is indicated for the treatment of ...Close
1.2 Episodic Cluster Headache
EMGALITY is indicated for the treatment of episodic cluster headache in adults.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing for Migraine - The recommended dosage of EMGALITY is 240 mg (two consecutive subcutaneous injections of 120 mg each) once as a loading dose, followed by monthly doses of ...
2.1 Recommended Dosing for Migraine
The recommended dosage of EMGALITY is 240 mg (two consecutive subcutaneous injections of 120 mg each) once as a loading dose, followed by monthly doses of 120 mg injected subcutaneously.
If a dose of EMGALITY is missed, administer as soon as possible. Thereafter, EMGALITY can be scheduled monthly from the date of the last dose.
2.2 Recommended Dosing for Episodic Cluster Headache
The recommended dosage of EMGALITY is 300 mg (three consecutive subcutaneous injections of 100 mg each) at the onset of the cluster period, and then monthly until the end of the cluster period.
If a dose of EMGALITY is missed during a cluster period, administer as soon as possible. Thereafter, EMGALITY can be scheduled monthly from the date of the last dose until the end of the cluster period.
Close2.3 Important Administration Instructions
EMGALITY is for subcutaneous use only.
EMGALITY is intended for patient self-administration. Prior to use, provide proper training to patients and/or caregivers on how to prepare and administer EMGALITY using the single-dose prefilled pen or single-dose prefilled syringe, including aseptic technique [see How Supplied/Storage and Handling (16.2) and Instructions for Use]:
- Protect EMGALITY from direct sunlight.
- Prior to subcutaneous administration, allow EMGALITY to sit at room temperature for 30 minutes. Do not warm by using a heat source such as hot water or a microwave.
- Do not shake the product.
- Inspect EMGALITY visually for particulate matter and discoloration prior to administration, whenever solution and container permit [see Dosage Forms and Strengths (3) and How Supplied/Storage and Handling (16.1)]. Do not use EMGALITY if it is cloudy or there are visible particles.
- Administer EMGALITY in the abdomen, thigh, back of the upper arm, or buttocks subcutaneously. Do not inject into areas where the skin is tender, bruised, red, or hard.
- Both the prefilled pen and prefilled syringe are single-dose and deliver the entire contents.
-
3 DOSAGE FORMS AND STRENGTHS
EMGALITY is a sterile clear to opalescent, colorless to slightly yellow to slightly brown solution available as follows: Injection: 120 mg/mL in a single-dose prefilled pen - Injection: 120 mg/mL ...
EMGALITY is a sterile clear to opalescent, colorless to slightly yellow to slightly brown solution available as follows:
- Injection: 120 mg/mL in a single-dose prefilled pen
- Injection: 120 mg/mL in a single-dose prefilled syringe
- Injection: 100 mg/mL in a single-dose prefilled syringe
-
4 CONTRAINDICATIONS
EMGALITY is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including dyspnea, urticaria, and rash, have occurred with EMGALITY in clinical studies and the postmarketing setting ...
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including dyspnea, urticaria, and rash, have occurred with EMGALITY in clinical studies and the postmarketing setting. Cases of anaphylaxis and angioedema have also been reported in the postmarketing setting. If a serious or severe hypersensitivity reaction occurs, discontinue administration of EMGALITY and initiate appropriate therapy [see Contraindications (4), Adverse Reactions (6.1), and Patient Counseling Information (17)]. Hypersensitivity reactions can occur days after administration and may be prolonged.
5.2 Hypertension
Development of hypertension and worsening of pre-existing hypertension have been reported following the use of CGRP antagonists, including EMGALITY, in the postmarketing setting. Some of the patients who developed new-onset hypertension had risk factors for hypertension. There were cases requiring initiation of pharmacological treatment for hypertension and, in some cases, hospitalization. Hypertension may occur at any time during treatment but was most frequently reported within 7 days of therapy initiation. EMGALITY was discontinued in many of the reported cases.
Monitor patients treated with EMGALITY for new-onset hypertension or worsening of pre-existing hypertension, and consider whether discontinuation of EMGALITY is warranted if evaluation fails to establish an alternative etiology or blood pressure is inadequately controlled.
Close5.3 Raynaud's Phenomenon
Development of Raynaud’s phenomenon and recurrence or worsening of pre-existing Raynaud’s phenomenon have been reported in the postmarketing setting following the use of CGRP antagonists, including EMGALITY. In reported cases with monoclonal antibody CGRP antagonists, symptom onset occurred after a median of 71 days following dosing. Many of the cases reported serious outcomes, including hospitalizations and disability, generally related to debilitating pain. In most reported cases, discontinuation of the CGRP antagonist resulted in resolution of symptoms.
EMGALITY should be discontinued if signs or symptoms of Raynaud’s phenomenon develop, and patients should be evaluated by a healthcare provider if symptoms do not resolve. Patients with a history of Raynaud’s phenomenon should be monitored for, and informed about the possibility of, worsening or recurrence of signs and symptoms.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions (5.1)]
- Hypertension [see Warnings and Precautions (5.2)]
- Raynaud's Phenomenon [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
Migraine
The safety of EMGALITY has been evaluated in 2586 patients with migraine who received at least one dose of EMGALITY, representing 1487 patient-years of exposure. Of these, 1920 patients were exposed to EMGALITY once monthly for at least 6 months, and 526 patients were exposed for 12 months.
In placebo-controlled clinical studies (Studies 1, 2, and 3), 705 patients received at least one dose of EMGALITY 120 mg once monthly, and 1451 patients received placebo, during 3 months or 6 months of double-blind treatment [see Clinical Studies (14.1)]. Of the EMGALITY-treated patients, approximately 85% were female, 77% were white, and the mean age was 41 years at study entry.
The most common adverse reaction was injection site reactions. In Studies 1, 2, and 3, 1.8% of patients discontinued double-blind treatment because of adverse events. Table 1 summarizes the adverse reactions that occurred within up to 6 months of treatment in the migraine studies.
Table 1: Adverse Reactions Occurring in Adults with Migraine with an Incidence of at least 2% for EMGALITY and at least 2% Greater than Placebo (up to 6 Months of Treatment) in Studies 1, 2, and 3 a Injection site reactions include multiple related adverse event terms, such as injection site pain, injection site reaction, injection site erythema, and injection site pruritus.
Adverse ReactionEMGALITY 120 mg
Monthly
(N=705)
%Placebo
Monthly
(N=1451)
%Injection site reactionsa 18 13 Episodic Cluster Headache
EMGALITY was studied for up to 2 months in a placebo-controlled trial in patients with episodic cluster headache (Study 4) [see Clinical Studies (14.2)]. A total of 106 patients were studied (49 on EMGALITY and 57 on placebo). Of the EMGALITY-treated patients, approximately 84% were male, 88% were white, and the mean age was 47 years at study entry. Two EMGALITY-treated patients discontinued double-blind treatment because of adverse events.
Overall, the safety profile observed in patients with episodic cluster headache treated with EMGALITY 300 mg monthly is consistent with the safety profile in migraine patients.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
For these reasons, comparison of the incidence of antibodies to galcanezumab-gnlm in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
The immunogenicity of EMGALITY has been evaluated using an in vitro immunoassay for the detection of binding anti-galcanezumab-gnlm antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro ligand-binding immunoassay was performed to detect neutralizing antibodies.
In controlled studies with EMGALITY up to 6 months (Study 1, Study 2, and Study 3), the incidence of anti-galcanezumab-gnlm antibody development was 4.8% (33/688) in patients receiving EMGALITY once monthly (32 out of 33 of whom had in vitro neutralizing activity). With 12 months of treatment in an open-label study, up to 12.5% (16/128) of EMGALITY-treated patients developed anti-galcanezumab-gnlm antibodies, most of whom tested positive for neutralizing antibodies.
Although anti-galcanezumab-gnlm antibody development was not found to affect the pharmacokinetics, safety or efficacy of EMGALITY in these patients, the available data are too limited to make definitive conclusions.
Close6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of EMGALITY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to EMGALITY exposure.
Immune System Disorders — Anaphylaxis, angioedema [see Contraindications (4) and Warnings and Precautions (5.1)].
Skin and Subcutaneous Tissue Disorders — Rash.
Vascular Disorders — Hypertension [see Warnings and Precautions (5.2)], Raynaud's Phenomenon [see Warnings and Precautions (5.3)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to EMGALITY during pregnancy. Healthcare providers are ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to EMGALITY during pregnancy. Healthcare providers are encouraged to register pregnant patients, or pregnant women may enroll themselves in the registry by calling 1-833-464-4724 or by contacting the company at www.migrainepregnancyregistry.com.
Risk Summary
There are no adequate data on the developmental risk associated with the use of EMGALITY in pregnant women. Administration of galcanezumab-gnlm to rats and rabbits during the period of organogenesis or to rats throughout pregnancy and lactation at plasma exposures greater than that expected clinically did not result in adverse effects on development (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively. The estimated rate of major birth defects (2.2% - 2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Data
Animal Data
When galcanezumab-gnlm was administered to female rats by subcutaneous injection in two studies (0, 30, or 100 mg/kg; 0 or 250 mg/kg) prior to and during mating and continuing throughout organogenesis, no adverse effects on embryofetal development were observed. The highest dose tested (250 mg/kg) was associated with a plasma exposure (Cave, ss) 38 or 18 times that in humans at the recommended human dose (RHD) for migraine (120 mg) or episodic cluster headache (300 mg), respectively. Administration of galcanezumab-gnlm (0, 30, or 100 mg/kg) by subcutaneous injection to pregnant rabbits throughout the period of organogenesis produced no adverse effects on embryofetal development. The higher dose tested was associated with a plasma Cave, ss 64 or 29 times that in humans at 120 mg or 300 mg, respectively.
Administration of galcanezumab-gnlm (0, 30, or 250 mg/kg) by subcutaneous injection to rats throughout pregnancy and lactation produced no adverse effects on pre- and postnatal development. The higher dose tested was associated with a plasma Cave, ss 34 or 16 times that in humans at 120 mg or 300 mg, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of galcanezumab-gnlm in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EMGALITY and any potential adverse effects on the breastfed infant from EMGALITY or from the underlying maternal condition.
Close8.5 Geriatric Use
Clinical studies of EMGALITY did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
-
11 DESCRIPTION
Galcanezumab-gnlm is a humanized IgG4 monoclonal antibody specific for calcitonin-gene related peptide (CGRP) ligand. Galcanezumab-gnlm is produced in Chinese Hamster Ovary (CHO) cells by ...
Galcanezumab-gnlm is a humanized IgG4 monoclonal antibody specific for calcitonin-gene related peptide (CGRP) ligand. Galcanezumab-gnlm is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology. Galcanezumab-gnlm is composed of two identical immunoglobulin kappa light chains and two identical immunoglobulin gamma heavy chains and has an overall molecular weight of approximately 147 kDa.
EMGALITY (galcanezumab-gnlm) injection is a sterile, preservative-free, clear to opalescent and colorless to slightly yellow to slightly brown solution, for subcutaneous use. EMGALITY is supplied in a 1 mL single-dose prefilled pen to deliver 120 mg of galcanezumab-gnlm or a 1 mL single-dose prefilled syringe to deliver 100 mg or 120 mg of galcanezumab-gnlm. Each mL of solution contains 100 mg or 120 mg of galcanezumab-gnlm; L-histidine (0.5 mg); L-histidine hydrochloride monohydrate (1.5 mg); Polysorbate 80 (0.5 mg); Sodium Chloride (8.8 mg); Water for Injection, USP. The pH range is 5.3 - 6.3.
Close -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Galcanezumab-gnlm is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor. 12.2 ...
12.1 Mechanism of Action
Galcanezumab-gnlm is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor.
12.2 Pharmacodynamics
There are no relevant data on the pharmacodynamic effects of galcanezumab-gnlm.
Close12.3 Pharmacokinetics
Galcanezumab-gnlm exhibits linear pharmacokinetics and exposure increases proportionally with doses between 1 and 600 mg.
A loading dose of 240 mg achieved the serum galcanezumab-gnlm steady-state concentration after the first dose. A dose of 300 mg monthly would achieve steady-state concentration after the fourth dose. The time to maximum concentration is 5 days, and the elimination half-life is 27 days.
There was no difference in pharmacokinetic parameters between healthy volunteers, patients with episodic or chronic migraine, and patients with episodic cluster headache.
Absorption
Following a subcutaneous dose of galcanezumab-gnlm, the time to maximum concentration was about 5 days.
Injection site location did not significantly influence the absorption of galcanezumab-gnlm.
Distribution
The apparent volume of distribution (V/F) of galcanezumab-gnlm was 7.3 L (34% Inter Individual Variability [IIV]).
Metabolism and Elimination
Galcanezumab-gnlm is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
The apparent clearance (CL/F) of galcanezumab-gnlm was 0.008 L/h and the elimination half-life of galcanezumab was approximately 27 days.
Specific Populations
Age, Sex, Weight, Race, Ethnicity
The pharmacokinetics of galcanezumab-gnlm were not affected by age, sex, race, subtypes of migraine spectrum (episodic or chronic migraine), or headache diagnosis (migraine vs. episodic cluster headache) based on a population pharmacokinetics analysis. Body weight has no clinically relevant effect on the pharmacokinetics of galcanezumab-gnlm.
Patients with Renal or Hepatic Impairment
Renal and hepatic impairment are not expected to affect the pharmacokinetics of galcanezumab-gnlm. Population pharmacokinetic analysis of integrated data from the galcanezumab-gnlm clinical studies revealed that creatinine clearance did not affect the pharmacokinetics of galcanezumab-gnlm in patients with mild or moderate renal impairment. Patients with severe renal impairment (creatinine clearance <30 mL/min) have not been studied. Based on a population PK analysis, bilirubin concentration did not significantly influence the CL/F of galcanezumab-gnlm.
No dedicated clinical studies were conducted to evaluate the effect of hepatic impairment or renal impairment on the pharmacokinetics of galcanezumab-gnlm.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of galcanezumab-gnlm has not been assessed. Mutagenesis - Genetic toxicology ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
When galcanezumab-gnlm (0, 30, or 250 mg/kg) was administered to male rats by subcutaneous injection prior to and during mating, no adverse effects on fertility was observed. The higher dose tested was associated with a plasma exposure (Cave, ss) 8 or 4 times that in humans at the recommended human dose (RHD) for migraine (120 mg) or episodic cluster headache (300 mg), respectively. When galcanezumab-gnlm was administered to female rats by subcutaneous injection in two studies (0, 30, or 100 mg/kg; 0 or 250 mg/kg) prior to and during mating and continuing throughout organogenesis, no adverse effects on fertility were observed. The highest dose tested (250 mg/kg) was associated with a plasma Cave, ss 38 or 18 times that in humans at 120 mg or 300 mg, respectively.
-
14 CLINICAL STUDIES
14.1 Migraine - The efficacy of EMGALITY was evaluated as a preventive treatment of episodic or chronic migraine in three multicenter, randomized, double-blind, placebo-controlled studies: two ...
14.1 Migraine
The efficacy of EMGALITY was evaluated as a preventive treatment of episodic or chronic migraine in three multicenter, randomized, double-blind, placebo-controlled studies: two 6-month studies in patients with episodic migraine (Studies 1 and 2) and one 3-month study in patients with chronic migraine (Study 3).
Episodic Migraine
Study 1 (NCT02614183) and Study 2 (NCT02614196) included adults with a history of episodic migraine (4 to 14 migraine days per month). All patients were randomized in a 1:1:2 ratio to receive once-monthly subcutaneous injections of EMGALITY 120 mg, EMGALITY 240 mg, or placebo. All patients in the 120 mg EMGALITY group received an initial 240 mg loading dose. Patients were allowed to use acute headache treatments, including migraine-specific medications (i.e., triptans, ergotamine derivatives), NSAIDs, and acetaminophen during the study.
The studies excluded patients on any other migraine preventive treatment, patients with medication overuse headache, patients with ECG abnormalities compatible with an acute cardiovascular event and patients with a history of stroke, myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, deep vein thrombosis, or pulmonary embolism within 6 months of screening.
The primary efficacy endpoint for Studies 1 and 2 was the mean change from baseline in the number of monthly migraine headache days over the 6-month treatment period. Key secondary endpoints included response rates (the mean percentages of patients reaching at least 50%, 75%, and 100% reduction from baseline in the number of monthly migraine headache days over the 6-month treatment period), the mean change from baseline in the number of monthly migraine headache days with use of any acute headache medication during the 6-month treatment period, and the impact of migraine on daily activities, as assessed by the mean change from baseline in the average Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ v2.1) Role Function-Restrictive domain score during the last 3 months of treatment (Months 4 to 6). Scores are scaled from 0 to 100, with higher scores indicating less impact of migraine on daily activities.
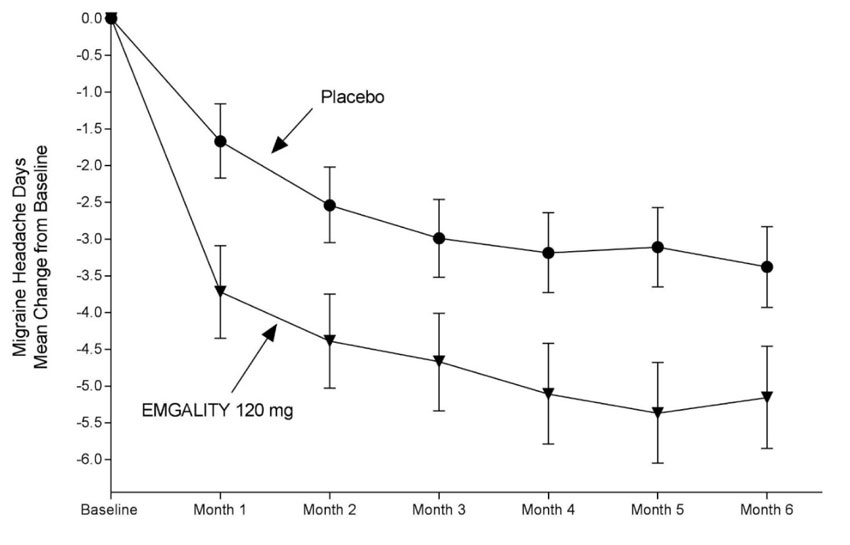
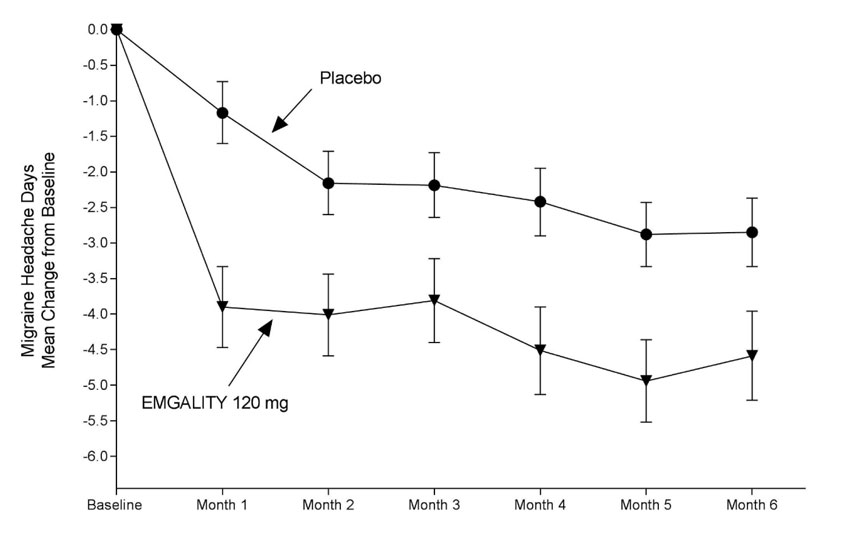
In Study 1, a total of 858 patients (718 females, 140 males) ranging in age from 18 to 65 years, were randomized. A total of 703 patients completed the 6-month double-blind phase. In Study 2, a total of 915 patients (781 female, 134 male) ranging in age from 18 to 65 years, were randomized. A total of 785 patients completed the 6-month double-blind phase. In Study 1 and Study 2, the mean migraine frequency at baseline was approximately 9 migraine days per month, and was similar across treatment groups.
EMGALITY 120 mg demonstrated statistically significant improvements for efficacy endpoints compared to placebo over the 6-month period, as summarized in Table 2. EMGALITY treatment with the 240 mg once-monthly dose showed no additional benefit over the EMGALITY 120 mg once-monthly dose.
Table 2: Efficacy Endpoints in Studies 1 and 2 a p<0.001
b N = 189 for EMGALITY 120 mg and N = 377 for placebo in Study 1; N = 213 for EMGALITY 120 mg and N = 396 for placebo in Study 2.
Study 1 Study 2 EMGALITY
120 mg
N = 210Placebo
N = 425EMGALITY
120 mg
N = 226Placebo
N = 450Monthly Migraine Headache Days (over Months 1 to 6) Baseline migraine headache days 9.2 9.1 9.1 9.2 Mean change from baseline -4.7 -2.8 -4.3 -2.3 Difference from placeboa -1.9 -2.0 ≥50% Migraine Headache Days Responders (over Months 1 to 6) % Respondersa 62% 39% 59% 36% ≥75% Migraine Headache Days Responders (over Months 1 to 6) % Respondersa 39% 19% 34% 18% 100% Migraine Headache Days Responders (over Months 1 to 6) % Respondersa 16% 6% 12% 6% Monthly Migraine Headache Days that Acute Medication was Taken (over Months 1 to 6) Mean change from baseline (days)a -4.0 -2.2 -3.7 -1.9 MSQ Role Function-Restrictive Domain Score (over Months 4 to 6) Baseline 51.4 52.9 52.5 51.4 Mean change from baselineb 32.4 24.7 28.5 19.7 Difference from placeboa 7.7 8.8 Figure 1: Change from Baseline in Monthly Migraine Headache Days in Study 1a
a Least-square means and 95% confidence intervals are presented.
Figure 2: Change from Baseline in Monthly Migraine Headache Days in Study 2a
a Least-square means and 95% confidence intervals are presented.
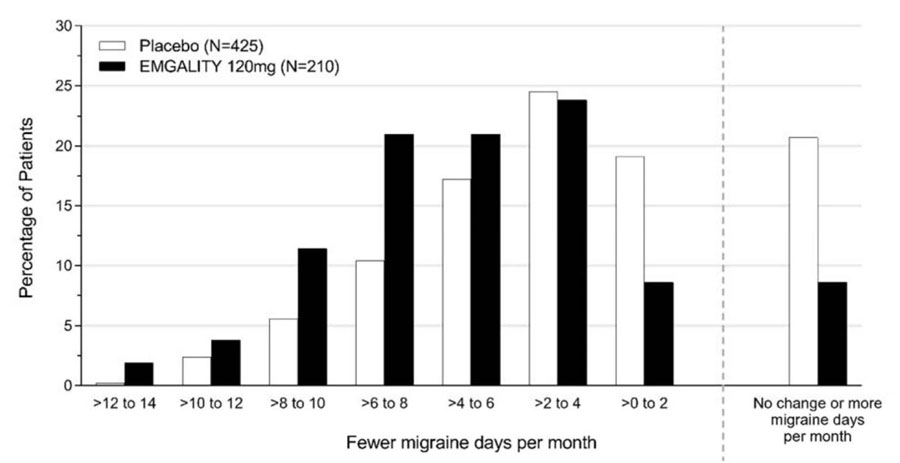
Figure 3 shows the distribution of change from baseline in the mean number of monthly migraine headache days in bins of 2 days, by treatment group, in Study 1. A treatment benefit over placebo for EMGALITY is seen across a range of changes from baseline in monthly migraine headache days.
Figure 3: Distribution of Change from Baseline in Mean Monthly Migraine Headache Days over Months 1 to 6 by Treatment Group in Study 1
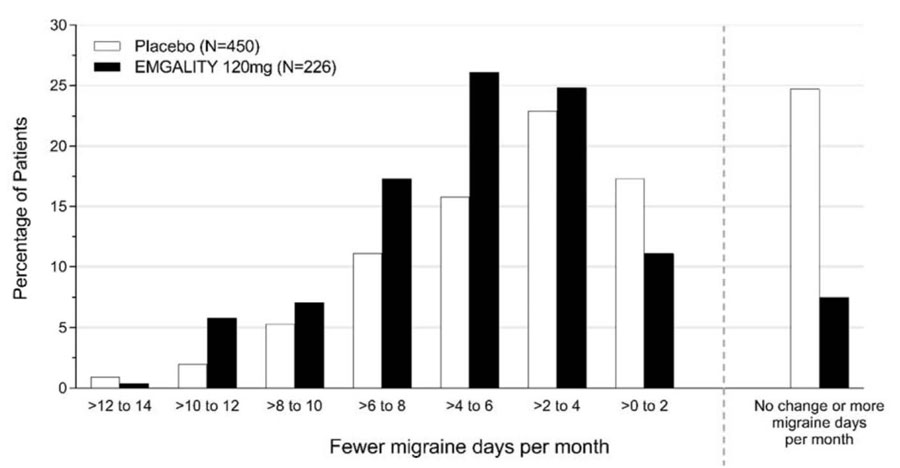
Figure 4 shows the distribution of change from baseline in the mean number of monthly migraine headache days in bins of 2 days, by treatment group, in Study 2. A treatment benefit over placebo for EMGALITY is seen across a range of changes from baseline in monthly migraine headache days.
Figure 4: Distribution of Change from Baseline in Mean Monthly Migraine Headache Days over Months 1 to 6 by Treatment Group in Study 2
Chronic Migraine
Study 3 (NCT02614261) included adults with a history of chronic migraine (≥15 headache days per month with ≥8 migraine days per month). All patients were randomized in a 1:1:2 ratio to receive once-monthly subcutaneous injections of EMGALITY 120 mg, EMGALITY 240 mg, or placebo over a 3-month treatment period. All patients in the 120 mg EMGALITY group received an initial 240 mg loading dose.
Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives), NSAIDs, and acetaminophen. A subset of patients (15%) was allowed to use one concomitant migraine preventive medication. Patients with medication overuse headache were allowed to enroll.
The study excluded patients with ECG abnormalities compatible with an acute cardiovascular event, and patients with a history of stroke, myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, deep vein thrombosis, or pulmonary embolism within 6 months of screening.
The primary endpoint was the mean change from baseline in the number of monthly migraine headache days over the 3-month treatment period. The secondary endpoints were response rates (the mean percentages of patients reaching at least 50%, 75% and 100% reduction from baseline in the number of monthly migraine headache days over the 3-month treatment period), the mean change from baseline in the number of monthly migraine headache days with use of any acute headache medication during the 3-month treatment period, and the impact of migraine on daily activities as assessed by the mean change from baseline in the MSQ v2.1 Role Function-Restrictive domain score at Month 3. Scores are scaled from 0 to 100, with higher scores indicating less impact of migraine on daily activities.
In Study 3, a total of 1113 patients (946 female, 167 male) ranging in age from 18 to 65 years, were randomized. A total of 1037 patients completed the 3-month double-blind phase. The mean number of monthly migraine headache days at baseline was approximately 19.
EMGALITY 120 mg demonstrated statistically significant improvement for the mean change from baseline in the number of monthly migraine headache days over the 3-month treatment period, and in the mean percentage of patients reaching at least 50% reduction from baseline in the number of monthly migraine headache days over the 3-month treatment period, as summarized in Table 3. EMGALITY treatment with the 240 mg once-monthly dose showed no additional benefit over the EMGALITY 120 mg once-monthly dose.
Table 3: Efficacy Endpoints in Study 3 a p<0.001
EMGALITY
120 mg
N = 273Placebo
N =538Monthly Migraine Headache Days (over Months 1 to 3) Baseline migraine headache days 19.4 19.6 Mean change from baseline -4.8 -2.7 Difference from placeboa -2.1 ≥50% Migraine Headache Days Responders (over Months 1 to 3) % Respondersa 28% 15% Study 3 utilized a sequential testing procedure to control the Type-I error rate for the multiple secondary endpoints. Once a secondary endpoint failed to reach the required level for statistical significance, formal hypothesis testing was terminated for subsequent endpoints, and p-values were considered nominal only. In Study 3, EMGALITY 120 mg was not significantly better than placebo for the proportion of patients with ≥75% or 100% reduction in migraine headache days. Patients treated with EMGALITY 120 mg showed a nominally greater reduction in the number of monthly migraine headache days that acute medication was taken (-4.7 for EMGALITY 120 mg vs. -2.2 for placebo; nominal p-value <0.001), and the mean change from baseline in the MSQ Role Function-Restrictive Domain score at Month 3 was nominally greater in patients treated with EMGALITY 120 mg than in patients on placebo (21.8 for EMGALITY 120 mg vs. 16.8 for placebo; nominal p-value <0.001).
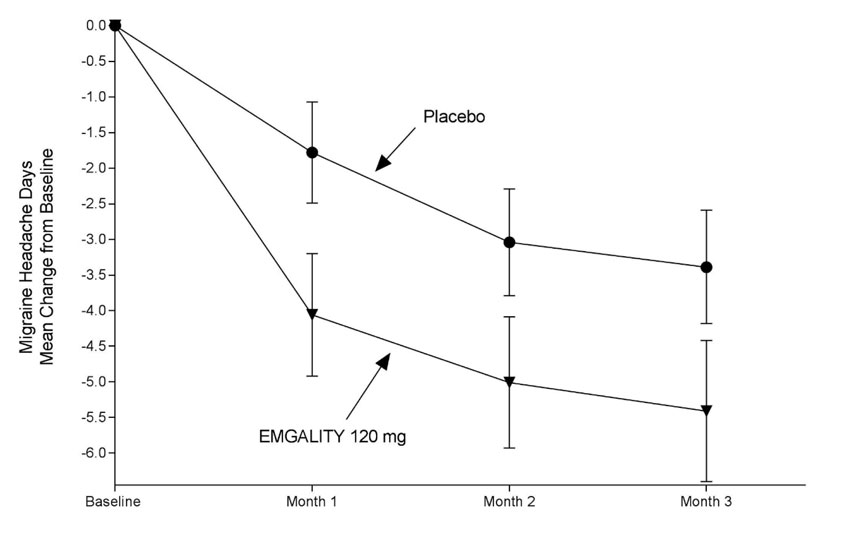
Figure 5: Change from Baseline in Monthly Migraine Headache Days in Study 3a
a Least-square means and 95% confidence intervals are presented.
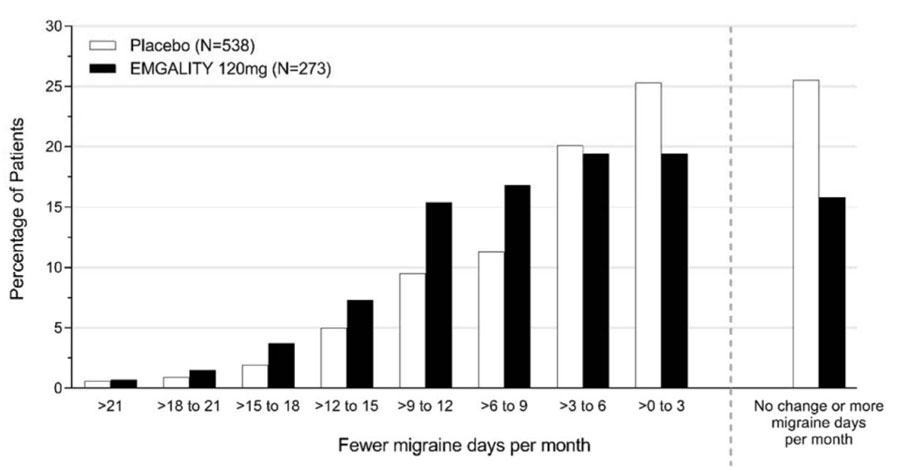
Figure 6 shows the distribution of change from baseline in the mean number of monthly migraine headache days for the 3-month study period in bins of 3 days by treatment group. A treatment benefit over placebo for EMGALITY is seen across a range of changes from baseline in monthly migraine headache days.
Figure 6: Distribution of Change from Baseline in Mean Monthly Migraine Headache Days over Months 1 to 3 by Treatment Group in Study 3
Close14.2 Episodic Cluster Headache
The efficacy of EMGALITY was evaluated for the treatment of episodic cluster headache in a randomized, 8-week, double-blind, placebo-controlled study (Study 4).
Study 4 (NCT02397473) included adults who met the International Classification of Headache Disorders 3rd edition (beta version) diagnostic criteria for episodic cluster headache and had a maximum of 8 attacks per day, a minimum of one attack every other day, and at least 4 attacks during the prospective 7-day baseline period. All patients were randomized in a 1:1 ratio to receive once-monthly subcutaneous injections of EMGALITY 300 mg or placebo. Patients were allowed to use certain specified acute/abortive cluster headache treatments, including triptans, oxygen, acetaminophen, and NSAIDs during the study.
The study excluded patients on other treatments intended to reduce the frequency of cluster headache attacks; patients with medication overuse headache; patients with ECG abnormalities compatible with an acute cardiovascular event or conduction delay; and patients with a history of myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, deep vein thrombosis, or pulmonary embolism within 6 months of screening. In addition, patients with any history of stroke, intracranial or carotid aneurysm, intracranial hemorrhage, or vasospastic angina; clinical evidence of peripheral vascular disease; or diagnosis of Raynaud’s disease were excluded.
The primary efficacy endpoint for Study 4 was the mean change from baseline in weekly cluster headache attack frequency across Weeks 1 to 3. A secondary endpoint was the percentage of patients who achieved a response (defined as a reduction from baseline of 50% or greater in the weekly cluster headache attack frequency) at Week 3.
In Study 4, a total of 106 patients (88 males, 18 females) ranging in age from 19 to 65 years were randomized and treated. A total of 90 patients completed the 8-week double-blind phase. In the prospective baseline phase, the mean number of weekly cluster headache attacks was 17.5, and was similar across treatment groups.
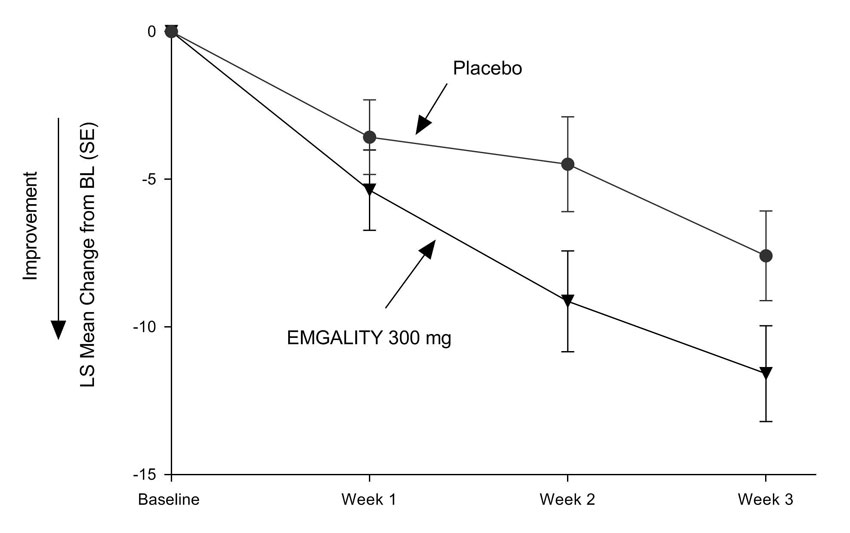
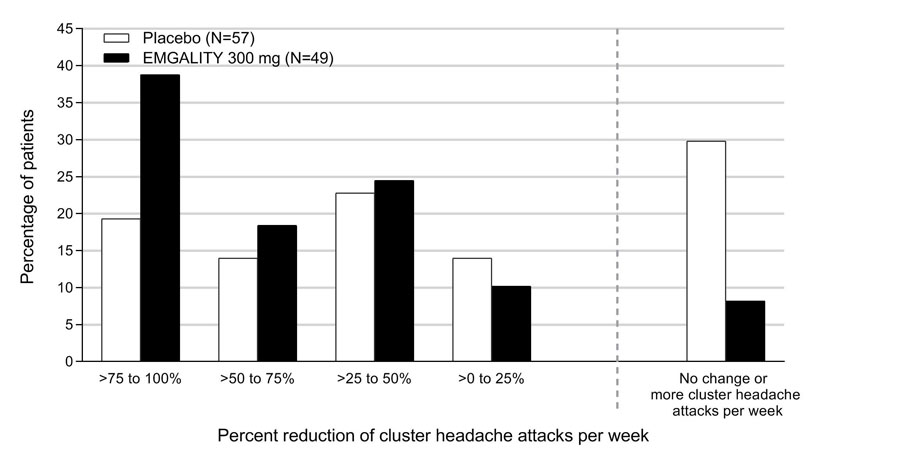
EMGALITY 300 mg demonstrated statistically significant improvements for efficacy endpoints compared to placebo, as summarized in Table 4.
Table 4: Efficacy Endpoints in Study 4 EMGALITY
300 mg
N = 49Placebo
N = 57Mean Reduction in Weekly Cluster Headache Attack Frequency (over Weeks 1 to 3) Prospective Baseline Cluster Headache Attack Frequency 17.8 17.3 Mean change from baseline -8.7 -5.2 Difference from placebo -3.5 p-value 0.036 ≥50% Weekly Cluster Headache Attack Frequency Responders (at Week 3) % Responders 71.4% 52.6% Difference from placebo 18.8% p-value 0.046 Figure 7: Mean Change in Weekly Cluster Headache Attack Frequency over Weeks 1 to 3 in Study 4a
a Abbreviations: BL = baseline; LS = least square; SE = standard error.
Figure 8 shows the distribution of the average percent change from baseline in weekly cluster headache attack frequency across Weeks 1 to 3 in bins of 25%, by treatment group, in Study 4.
Figure 8: Distribution of the Average Percent Change from Baseline in Weekly Cluster Headache Attack Frequency over Weeks 1 to 3 in Study 4a
a N = number of intent to treat patients with non-missing average percentage change from baseline in weekly cluster headache attack frequency over weeks 1 to 3.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - EMGALITY (galcanezumab-gnlm) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous ...
16.1 How Supplied
EMGALITY (galcanezumab-gnlm) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous administration.
EMGALITY is not made with natural rubber latex.
EMGALITY is supplied as follows:
Pack Size NDC Prefilled pen 120 mg/mL single-dose Carton of 1 0002-1436-11 120 mg/mL single-dose Carton of 2 0002-1436-27 Prefilled syringe 100 mg/mL single-dose Carton of 3 0002-3115-09 120 mg/mL single-dose Carton of 1 0002-2377-11 120 mg/mL single-dose Carton of 2 0002-2377-27 Close16.2 Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect EMGALITY from light until use.
- Do not freeze.
- Do not shake.
- EMGALITY may be stored out of refrigeration in the original carton at temperatures up to 30°C (86°F) for up to 7 days. Once stored out of refrigeration, do not place back in the refrigerator.
- If these conditions are exceeded, EMGALITY must be discarded.
- Discard the EMGALITY single-dose prefilled pen or syringe after use in a puncture-resistant container.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Instructions on Self-Administration: Provide guidance to patients and/or caregivers ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Instructions on Self-Administration: Provide guidance to patients and/or caregivers on proper subcutaneous injection technique, including aseptic technique, and how to use the prefilled pen or prefilled syringe correctly [see Instructions for Use]. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use EMGALITY.
Hypersensitivity Reactions:
Inform patients about the signs and symptoms of hypersensitivity reactions and that these reactions can occur with EMGALITY. Advise patients to seek immediate medical attention if they experience any symptoms of serious or severe hypersensitivity reactions [see Warnings and Precautions (5.1)].
Hypertension:
Inform patients that hypertension can develop or pre-existing hypertension can worsen with EMGALITY, and that they should contact their healthcare provider if they experience elevation in their blood pressure [see Warnings and Precautions (5.2)].
Raynaud's Phenomenon:
Inform patients that Raynaud's phenomenon can develop or worsen with EMGALITY. Advise patients to discontinue EMGALITY and contact their healthcare provider if they experience signs or symptoms of Raynaud's phenomenon [see Warnings and Precautions (5.3)].
Pregnancy Exposure Registry: Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to EMGALITY during pregnancy [see Use in Specific Populations (8.1)].
For more information go to www.emgality.com or call 1-833-EMGALITY (1-833-364-2548).
Literature revised: 03/2025
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License Number 1891
Copyright © 2018, 2025, Eli Lilly and Company. All rights reserved.
EMG-0007-USPI-20250321
Close -
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration - Revised: 03/2025 - PATIENT INFORMATION - EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection ...Close
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 03/2025
PATIENT INFORMATION
EMGALITY® (em-GAL-it-ē)
(galcanezumab-gnlm)
injection, for subcutaneous useWhat is EMGALITY?
EMGALITY is a prescription medicine used in adults for the:
- preventive treatment of migraine.
- treatment of episodic cluster headache.
It is not known if EMGALITY is safe and effective in children. Who should not use EMGALITY?
Do not use EMGALITY if you are allergic to galcanezumab-gnlm or any of the ingredients in EMGALITY. See the end of this Patient Information for a complete list of ingredients in EMGALITY.Before you use EMGALITY, tell your healthcare provider if you:
- have high blood pressure.
- have circulation problems in your fingers and toes.
- are pregnant or plan to become pregnant. It is not known if EMGALITY will harm your unborn baby.
- Pregnancy Registry: There is a pregnancy registry for women who take EMGALITY. The purpose of this registry is to collect information about the health of you and your baby. You may enroll yourself by calling 1-833-464-4724 or by visiting www.migrainepregnancyregistry.com. Or you may talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. It is not known if EMGALITY passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using EMGALITY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine. How should I use EMGALITY?
- See the Instructions for Use that come with EMGALITY prefilled pen or prefilled syringe about how to use EMGALITY the right way.
- Use EMGALITY exactly as your healthcare provider tells you to.
- EMGALITY is given by injection under the skin (subcutaneous injection).
- Inject EMGALITY in your stomach area (abdomen), thigh, back of the upper arm, or buttocks.
- Your healthcare provider should show you or a caregiver how to prepare and inject EMGALITY the right way before you start to use it.
- EMGALITY comes in 2 different types of devices:
- a single-dose (1 time) prefilled pen
- a single-dose (1 time) prefilled syringe
Your healthcare provider will prescribe the type that is best for you.
- If you have questions about injecting the medicine, talk to your pharmacist or healthcare provider.
-
If you are using the EMGALITY 120 mg prefilled pen or prefilled syringe for migraine:
- Inject EMGALITY 1 time each month.
- For the first dose (loading dose), you will get 2 separate injections one time, right after each other. You will need 2 prefilled pens or 2 prefilled syringes for your first dose (1-time loading dose).
- For your regular monthly dose, you will get 1 injection. You will need 1 prefilled pen or 1 prefilled syringe for your regular monthly dose.
- If you miss a dose of EMGALITY, inject the missed dose as soon as possible. Then inject EMGALITY 1 month from the date of your last dose to get back on a monthly dosing schedule. If you have questions about your schedule, ask your healthcare provider.
-
If you are using the EMGALITY 100 mg prefilled syringe for episodic cluster headache:
- You will get 3 separate injections, right after each other, using 3 prefilled syringes for each of your doses.
- Use EMGALITY at the start of a cluster period and then every month until the end of the cluster period.
- If you miss a dose of EMGALITY, inject the missed dose as soon as possible. Then, if the cluster headache period has not yet ended, inject EMGALITY 1 month after your last dose to get back on a monthly dosing schedule. If you have questions about when you should use EMGALITY, ask your healthcare provider.
What are the possible side effects of EMGALITY?
EMGALITY may cause serious side effects, including:
-
Allergic reactions. Allergic reactions, including itching, rash, hives, and trouble breathing, can happen after receiving EMGALITY. This can happen days after using EMGALITY. Call your healthcare provider or get emergency medical help right away if you have any of the following symptoms, which may be part of an allergic reaction:
- swelling of the face, mouth, tongue, or throat
- trouble breathing
- High blood pressure. High blood pressure or worsening of high blood pressure can happen after receiving EMGALITY. Contact your healthcare provider if you have an increase in blood pressure.
- Raynaud’s phenomenon. A type of circulation problem can worsen or happen after receiving EMGALITY. Raynaud’s phenomenon can lead to your fingers or toes feeling numb, cool, or painful, or changing color from pale, to blue, to red. Contact your healthcare provider if these symptoms occur.
The most common side effects of EMGALITY include:
- injection site reactions
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of EMGALITY. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store EMGALITY?
- Store EMGALITY in the refrigerator between 36°F to 46°F (2°C to 8°C).
- EMGALITY may be stored out of the refrigerator in the original carton at temperatures up to 86°F (30°C) for up to 7 days. After storing out of the refrigerator, do not place EMGALITY back in the refrigerator.
- Do not freeze EMGALITY.
- Keep EMGALITY in the carton it comes in to protect it from light until time of use.
- Do not shake EMGALITY.
- Throw away EMGALITY if any of the above conditions are not followed.
Keep EMGALITY and all medicines out of the reach of children. General information about the safe and effective use of EMGALITY.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use EMGALITY for a condition for which it was not prescribed. Do not give EMGALITY to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about EMGALITY that is written for health professionals.What are the ingredients in EMGALITY?
Active ingredient: galcanezumab-gnlm
Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, Polysorbate 80, Sodium Chloride, and Water for Injection, USP.
EMGALITY prefilled pen and prefilled syringes are not made with natural rubber latex.
EMGALITY® is a registered trademark of Eli Lilly and Company.
Eli Lilly and Company Indianapolis, IN 46285, USA
US License Number 1891
Copyright © 2018, 2025, Eli Lilly and Company. All rights reserved.
EMG-0006-PPI-20250321
For more information, call 1-833-EMGALITY (1-833-364-2548) or go to www.emgality.com. -
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use - Prefilled Pen - This Instructions for Use is for patients with ...
INSTRUCTIONS FOR USE EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Pen

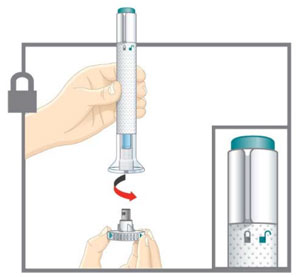
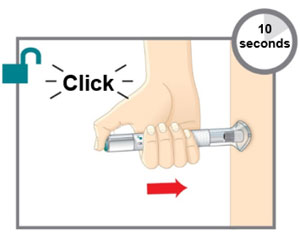
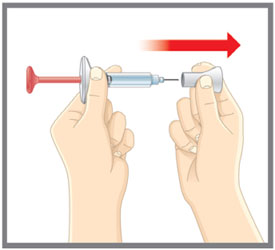
This Instructions for Use is for patients with migraine. For subcutaneous injection only. Before you use the EMGALITY prefilled pen (Pen), read and carefully follow all the step-by-step instructions. Important Information - Your healthcare provider or nurse should show you how to prepare and inject EMGALITY using the Pen. Do not inject yourself or someone else until you have been shown how to inject EMGALITY.
- Keep this Instructions for Use and refer to it as needed.
- Each EMGALITY Pen is for one-time use only. Do not share or reuse your EMGALITY Pen. You may give or get an infection.
- The Pen contains glass parts. Handle it carefully. If you drop it on a hard surface, do not use it. Use a new Pen for your injection.
- Your healthcare provider may help you decide where on your body to inject your dose. You can also read the “Choose your injection site” section of these instructions to help you choose which area can work best for you.
- If you have vision or hearing problems, do not use EMGALITY Pen without help from a caregiver.
- See “Storage and Handling Information” for important storage information.
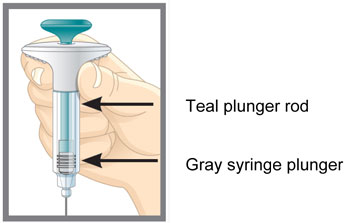
INSTRUCTIONS FOR USE Before you use the EMGALITY Pen, read and carefully follow all the step-by-step instructions. Parts of the EMGALITY Pen
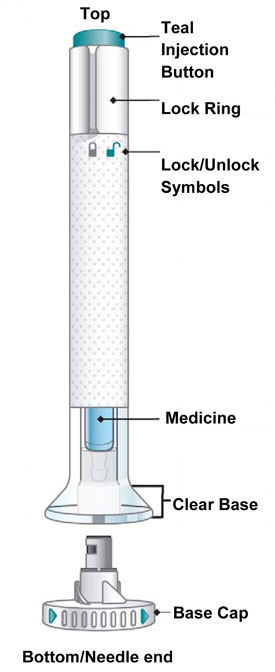
Before You Get Started Take the Pen from the refrigerator Check your prescription. - EMGALITY comes as a single-dose prefilled Pen.
- You will need 2 Pens for your first dose (1-time loading dose). You will need 1 Pen for your monthly dose.
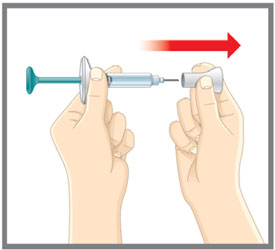
Put the original package with any unused Pens back in the refrigerator.Leave the base cap on until you are ready to inject. Leave the Pen at room temperature for 30 minutes before injecting. Do not microwave the Pen, run hot water over it, or leave it in direct sunlight. Do not shake. Gather Supplies For each injection you will need:
- 1 alcohol wipe
- 1 cotton ball or piece of gauze
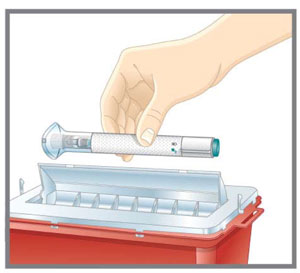
- 1 sharps disposal container. See “After You Inject Your Medicine.”
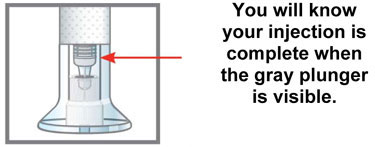
Inspect the Pen and the medicine Make sure you have the right medicine. The medicine inside should be clear. Its color may be colorless to slightly yellow to slightly brown.
Do not use the Pen, and throw away (dispose of) as directed by your healthcare provider or pharmacist if:
- it looks damaged
- the medicine is cloudy, is discolored, or has small particles
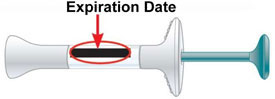
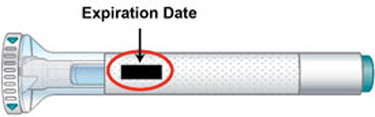
- the Expiration Date (Exp.) printed on the label has passed
- the medicine is frozen
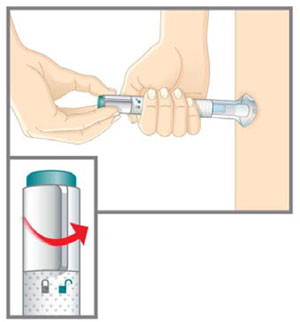
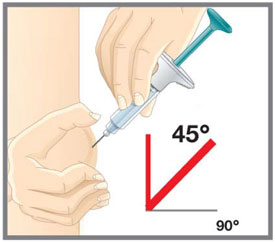
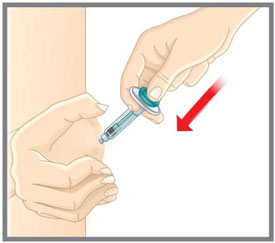
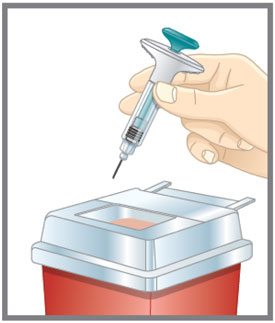
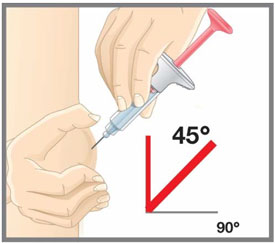
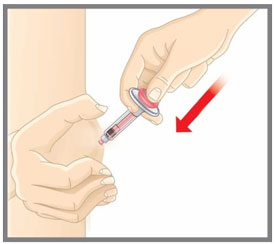
Prepare for injection Wash your hands with soap and water before you inject your EMGALITY. Make sure a sharps disposal container is close by. Your healthcare provider can help you choose the injection site that is best for you.
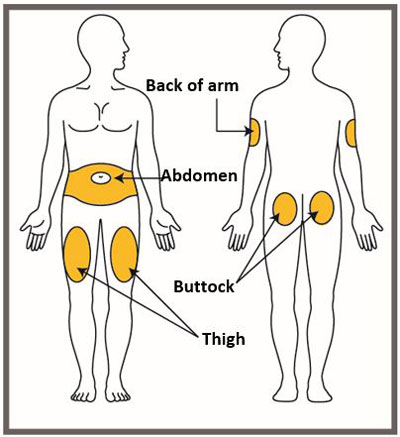
- You may inject the medicine into your stomach area (abdomen). Do not inject within 2 inches of the belly button (navel).
- You may inject the medicine into the front of your thighs. This area should be at least 2 inches above the knee and 2 inches below the groin.
- Another person may give you the injection in the back of your upper arm, or buttocks.
- Do not inject in the exact same spot. For example, if you are giving 2 injections for your first dose (1-time loading dose) and want to use the same body site for the two separate injections, choose a different injection spot. If your first injection was in your abdomen, your next injection could be in another area of your abdomen.
- Do not inject into areas where the skin is tender, bruised, red, or hard.
- Clean your injection site with an alcohol wipe. Let the injection site dry before you inject.
- Store your Pen in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- Your Pen may be stored out of the refrigerator in the original carton at temperatures up to 86°F (30°C) for up to 7 days. After storing out of the refrigerator, do not place EMGALITY back in the refrigerator.
- Do not freeze your Pen.
- Keep your Pen in the carton it comes in to protect it from light until time of use.
- Do not shake your Pen.
- Throw away your Pen if any of the above conditions are not followed.
- Keep your Pen and all medicines out of the reach of children.
Read the full Prescribing Information and Patient Information for EMGALITY inside this box to learn more about your medicine. Close
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Eli Lilly and Company
Indianapolis, IN 46285, USAUS License Number 1891
EMGALITY® is a registered trademark of Eli Lilly and Company.
Copyright © 2018, 2019, Eli Lilly and Company. All rights reserved.
Revised: 11/2019
The Pen meets the current dose accuracy and functional requirements of ISO 11608-1 and 11608-5.
EMG-0005-AI-120MG-IFU-20191127 -
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use - Prefilled Syringe - This Instructions for Use is for patients with ...
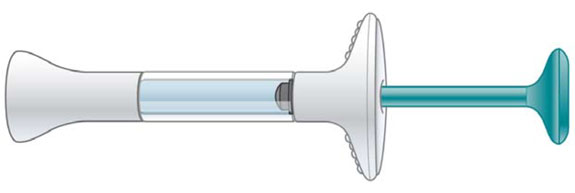
INSTRUCTIONS FOR USE EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Syringe
This Instructions for Use is for patients with migraine. - If you are using EMGALITY for episodic cluster headache, there is a different Instructions for Use because the dose and number of syringes needed is different.
For subcutaneous injection only. Before you use the EMGALITY prefilled syringe, read and carefully follow all the step-by-step instructions. Important Information - Your healthcare provider or nurse should show you how to prepare and inject EMGALITY using the prefilled syringe. Do not inject yourself or someone else until you have been shown how to inject EMGALITY.
- Keep this Instructions for Use and refer to it as needed.
- Each EMGALITY prefilled syringe is for one-time use only. Do not share or reuse your EMGALITY prefilled syringe. You may give or get an infection.
- Your healthcare provider may help you decide where on your body to inject your dose. You can also read the “Choose your injection site” section of these instructions to help you choose which area can work best for you.
- If you have vision problems, do not use EMGALITY prefilled syringe without help from a caregiver.
- See “Storage and Handling Information” for important storage information.
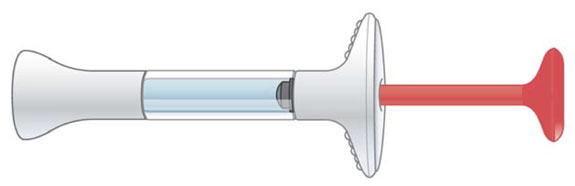
INSTRUCTIONS FOR USE Before you use the EMGALITY prefilled syringe, read and carefully follow all the step-by-step instructions. Parts of the EMGALITY Prefilled Syringe
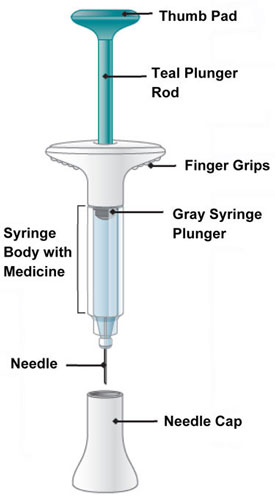
Storage and Handling Information - Store your prefilled syringe in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- Your prefilled syringe may be stored out of the refrigerator in the original carton at temperatures up to 86°F (30°C) for up to 7 days. After storing out of the refrigerator, do not place EMGALITY back in the refrigerator.
- Do not freeze your prefilled syringe.
- Keep your prefilled syringe in the carton it comes in to protect it from light until time of use.
- Do not shake your prefilled syringe.
- Throw away your prefilled syringe if any of the above conditions are not followed.
- Keep your prefilled syringe and all medicines out of the reach of children.
Read the full Prescribing Information and Patient Information for EMGALITY inside this box to learn more about your medicine.Close
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Eli Lilly and Company Indianapolis, IN 46285, USA US License Number 1891
EMGALITY® is a registered trademark of Eli Lilly and Company.
Copyright © 2018, 2019, Eli Lilly and Company. All rights reserved.
Revised: 06/2019
EMG-0004-PFS-120MG-IFU-20190604 -
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use - Prefilled Syringe - This Instructions for Use is for patients with episodic ...
INSTRUCTIONS FOR USE EMGALITY® (em-GAL-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Syringe
This Instructions for Use is for patients with episodic cluster headache. - If you are using EMGALITY for preventive treatment of migraine, there is a different Instructions for Use because the dose and number of syringes needed is different.
For subcutaneous injection only. Before you use the EMGALITY prefilled syringe, read and carefully follow all the step-by-step instructions. Important Information - Your healthcare provider or nurse should show you how to prepare and inject EMGALITY using the prefilled syringe. Do not inject yourself or someone else until you have been shown how to inject EMGALITY.
- Keep this Instructions for Use and refer to it as needed.
- Each EMGALITY prefilled syringe is for one-time use only. Do not share or reuse your EMGALITY prefilled syringe. You may give or get an infection.
- Your healthcare provider may help you decide where on your body to inject your dose. You can also read the “Choose your injection site” section of these instructions to help you choose which area can work best for you.
- If you have vision problems, do not use EMGALITY prefilled syringe without help from a caregiver.
- See “Storage and Handling Information” for important storage information.
INSTRUCTIONS FOR USE Before you use the EMGALITY prefilled syringe, read and carefully follow all the step-by-step instructions. Parts of the EMGALITY Prefilled Syringe
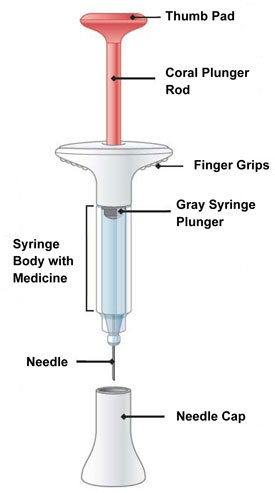
Before You Get Started Take the Prefilled Syringes from the refrigerator Take 3 EMGALITY prefilled syringes from the refrigerator.
Check your prescription.
- EMGALITY comes as a single-dose prefilled syringe.
- You will need 3 prefilled syringes for each dose.
Leave the needle caps on until you are ready to inject. Leave the prefilled syringes at room temperature for 30 minutes before injecting. Do not microwave the prefilled syringes, run hot water over them, or leave them in direct sunlight. Do not shake. Gather Supplies For each injection you will need:
- 1 alcohol wipe
- 1 cotton ball or piece of gauze
- 1 sharps disposal container. See “After You Inject Your Medicine.”
Inspect the Prefilled Syringe and the medicine Make sure you have the right medicine. The medicine inside should be clear. Its color may be colorless to slightly yellow to slightly brown. Do not use the prefilled syringe, and throw away (dispose of) as directed by your healthcare provider or pharmacist if:
- it looks damaged
- the medicine is cloudy, is discolored, or has small particles
- the Expiration Date (Exp.) printed on the label has passed
- the medicine is frozen
Expiration Date
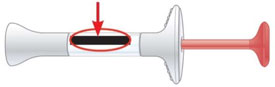
Prepare for injection Wash your hands with soap and water before you inject your EMGALITY. Make sure a sharps disposal container is close by. - Store your prefilled syringes in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- Your prefilled syringes may be stored out of the refrigerator in the original carton at temperatures up to 86ºF (30ºC) for up to 7 days. After storing out of the refrigerator, do not place EMGALITY back in the refrigerator.
- Do not freeze your prefilled syringes.
- Keep your prefilled syringes in the carton they come in to protect them from light until time of use.
- Do not shake your prefilled syringes.
- Throw away your prefilled syringes if any of the above conditions are not followed.
- Keep your prefilled syringes and all medicines out of the reach of children.
Read the full Prescribing Information and Patient Information for EMGALITY inside this box to learn more about your medicine. Close
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Eli Lilly and Company Indianapolis, IN 46285, USA US License Number 1891
EMGALITY® is a registered trademark of Eli Lilly and Company.
Copyright © 2019, Eli Lilly and Company. All rights reserved.
Issued: 06/2019
EMG-0001-PFS-100MG-IFU-20190604 -
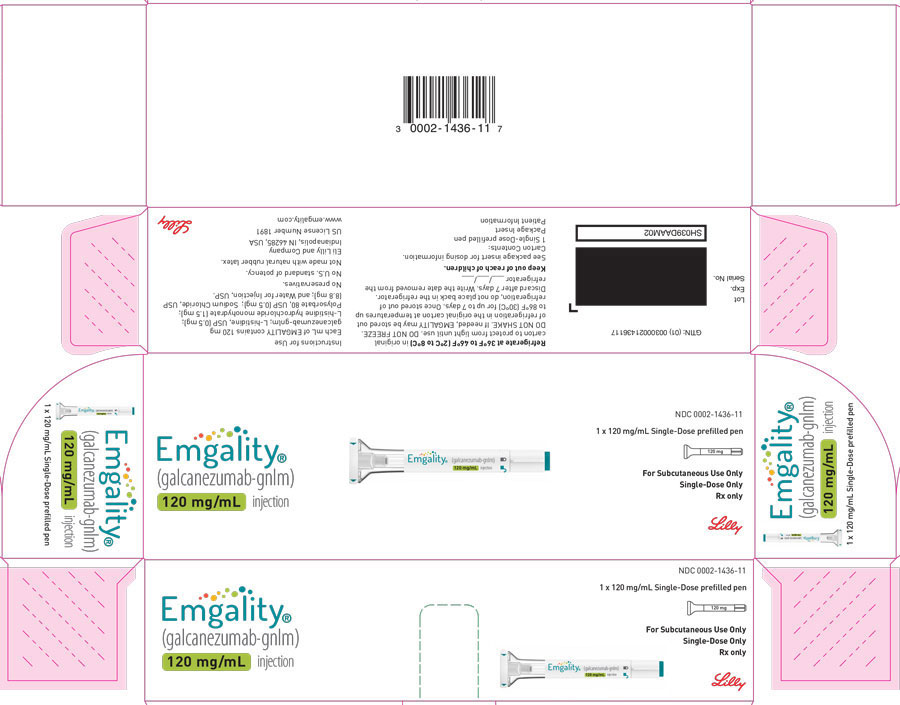
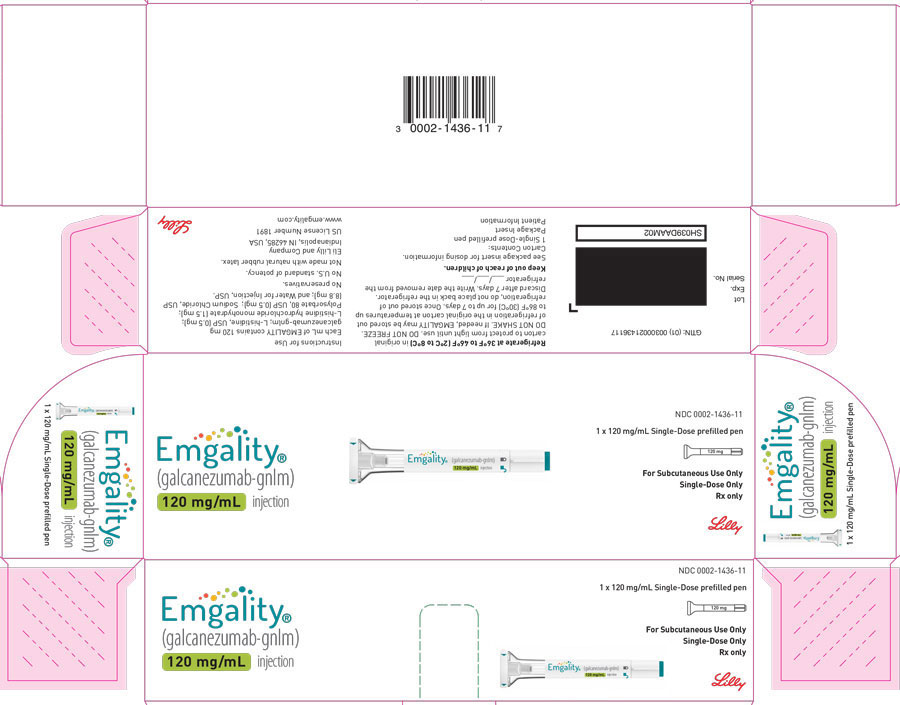
PRINCIPAL DISPLAY PANELPACKAGE CARTON – EMGALITY Autoinjector 120 mg - NDC 0002-1436-11 - EMGALITY® (galcanezumab-gnlm) injection - 120 mg/mL - 1 x 120 mg/mL Single-Dose prefilled pen - For Subcutaneous Use ...
PACKAGE CARTON – EMGALITY Autoinjector 120 mg
NDC 0002-1436-11
EMGALITY®
(galcanezumab-gnlm) injection
120 mg/mL
1 x 120 mg/mL Single-Dose prefilled pen
For Subcutaneous Use Only
Single-Dose Only
Rx only
Lilly
Close -
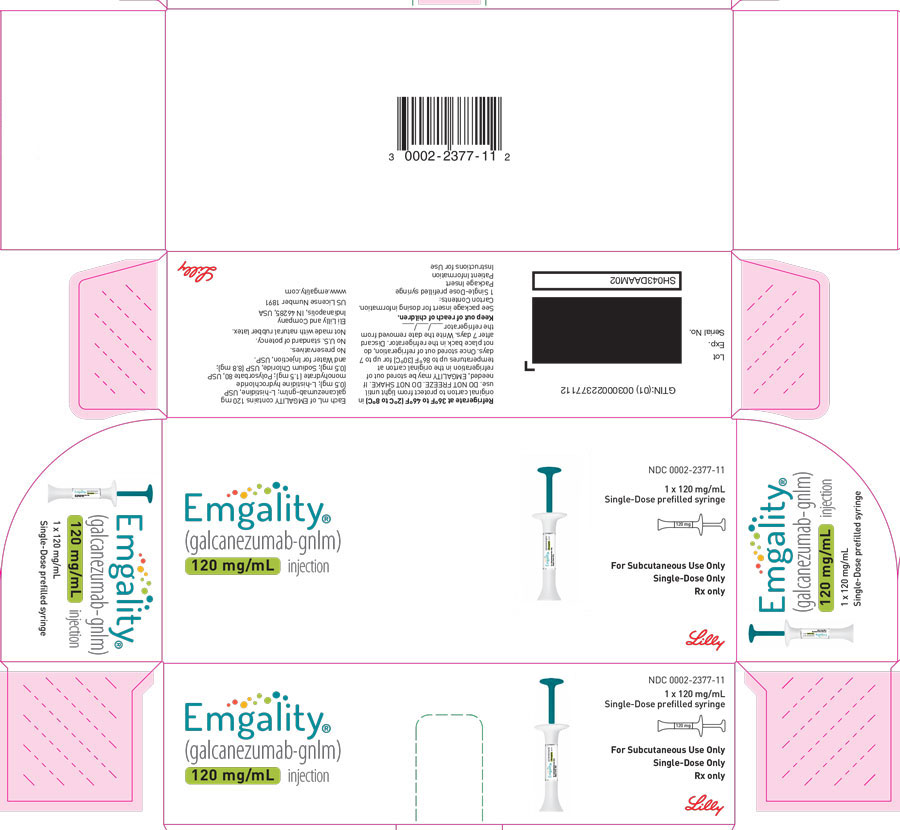
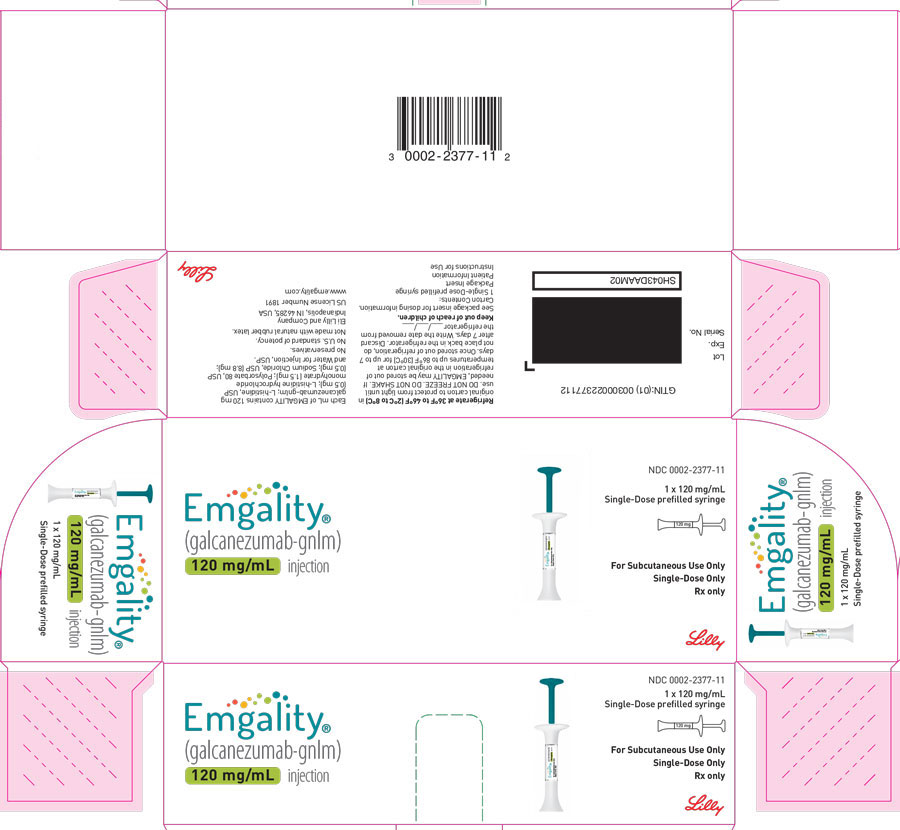
PRINCIPAL DISPLAY PANELPACKAGE CARTON – EMGALITY Prefilled Syringe 120 mg - NDC 0002-2377-11 - EMGALITY® (galcanezumab-gnlm) injection - 120 mg/mL - 1x 120 mg/mL Single-Dose prefilled syringe - For Subcutaneous Use ...
PACKAGE CARTON – EMGALITY Prefilled Syringe 120 mg
NDC 0002-2377-11
EMGALITY®
(galcanezumab-gnlm) injection
120 mg/mL
1x 120 mg/mL Single-Dose prefilled syringe
For Subcutaneous Use Only
Single-Dose Only
Rx only
Lilly
Close -
PRINCIPAL DISPLAY PANELPACKAGE CARTON – EMGALITY Prefilled Syringe 100 mg - NDC 0002-3115-09 - EMGALITY® (galcanezumab-gnlm) 100 mg/mL injection - All 3 syringes must be administered to receive the 300 mg dose. 3 x 100 ...
PACKAGE CARTON – EMGALITY Prefilled Syringe 100 mg
NDC 0002-3115-09
EMGALITY®
(galcanezumab-gnlm)
100 mg/mL injection
All 3 syringes must be administered to receive the 300 mg dose.
3 x 100 mg/mL Single-Dose prefilled syringe
For Subcutaneous Use Only
Single-Dose Only
Rx only
Lilly
Close -
INGREDIENTS AND APPEARANCEProduct Information
EMGALITY galcanezumab-gnlm injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-1436 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength galcanezumab (UNII: 55KHL3P693) (galcanezumab - UNII:55KHL3P693) galcanezumab 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength Histidine (UNII: 4QD397987E) 0.5 mg in 1 mL Histidine Monohydrochloride Monohydrate (UNII: X573657P6P) 1.5 mg in 1 mL Sodium Chloride (UNII: 451W47IQ8X) 8.8 mg in 1 mL Polysorbate 80 (UNII: 6OZP39ZG8H) 0.5 mg in 1 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-1436-11 1 in 1 CARTON 09/27/2018 1 NDC:0002-1436-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0002-1436-27 2 in 1 CARTON 09/27/2018 09/27/2018 2 NDC:0002-1436-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC:0002-1436-61 2 in 1 CARTON 09/27/2018 3 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761063 09/27/2018 EMGALITY galcanezumab-gnlm injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-2377 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength galcanezumab (UNII: 55KHL3P693) (galcanezumab - UNII:55KHL3P693) galcanezumab 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength Histidine (UNII: 4QD397987E) 0.5 mg in 1 mL Histidine Monohydrochloride Monohydrate (UNII: X573657P6P) 1.5 mg in 1 mL Sodium Chloride (UNII: 451W47IQ8X) 8.8 mg in 1 mL Polysorbate 80 (UNII: 6OZP39ZG8H) 0.5 mg in 1 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-2377-11 1 in 1 CARTON 01/18/2019 1 NDC:0002-2377-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0002-2377-27 2 in 1 CARTON 09/27/2018 09/27/2018 2 NDC:0002-2377-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761063 09/27/2018 EMGALITY galcanezumab-gnlm injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0002-3115 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength galcanezumab (UNII: 55KHL3P693) (galcanezumab - UNII:55KHL3P693) galcanezumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Histidine (UNII: 4QD397987E) 0.5 mg in 1 mL Histidine Monohydrochloride Monohydrate (UNII: X573657P6P) 1.5 mg in 1 mL Sodium Chloride (UNII: 451W47IQ8X) 8.8 mg in 1 mL Polysorbate 80 (UNII: 6OZP39ZG8H) 0.5 mg in 1 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0002-3115-09 3 in 1 CARTON 06/17/2019 1 NDC:0002-3115-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761063 06/04/2019
CloseLabeler - Eli Lilly and Company (006421325)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
EMGALITY- galcanezumab-gnlm injection, solution
Number of versions: 9
RxNorm
EMGALITY- galcanezumab-gnlm injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2058872 | galcanezumab-gnlm 120 MG in 1 ML Auto-Injector | PSN |
| 2 | 2058872 | 1 ML galcanezumab-gnlm 120 MG/ML Auto-Injector | SCD |
| 3 | 2058872 | galcanezumab-gnlm 120 MG per 1 ML Auto-Injector | SY |
| 4 | 2058877 | Emgality 120 MG in 1 ML Auto-Injector | PSN |
| 5 | 2058877 | 1 ML galcanezumab-gnlm 120 MG/ML Auto-Injector [Emgality] | SBD |
| 6 | 2058877 | Emgality 120 MG per 1 ML Auto-Injector | SY |
| 7 | 2058885 | galcanezumab-gnlm 120 MG in 1 ML Prefilled Syringe | PSN |
| 8 | 2058885 | 1 ML galcanezumab-gnlm 120 MG/ML Prefilled Syringe | SCD |
| 9 | 2058885 | galcanezumab-gnlm 120 MG per 1 ML Prefilled Syringe | SY |
| 10 | 2058887 | Emgality 120 MG in 1 ML Prefilled Syringe | PSN |
| 11 | 2058887 | 1 ML galcanezumab-gnlm 120 MG/ML Prefilled Syringe [Emgality] | SBD |
| 12 | 2058887 | Emgality 120 MG per 1 ML Prefilled Syringe | SY |
| 13 | 2170611 | galcanezumab-gnlm 100 MG in 1 ML Prefilled Syringe | PSN |
| 14 | 2170611 | 1 ML galcanezumab-gnlm 100 MG/ML Prefilled Syringe | SCD |
| 15 | 2170613 | Emgality 100 MG in 1 ML Prefilled Syringe | PSN |
| 16 | 2170613 | 1 ML galcanezumab-gnlm 100 MG/ML Prefilled Syringe [Emgality] | SBD |
| 17 | 2170613 | 1 ML Emgality 100 MG/ML Prefilled Syringe | SY |
| 18 | 2170613 | Emgality 100 MG per 1 ML Prefilled Syringe | SY |
Get Label RSS Feed for this Drug
EMGALITY- galcanezumab-gnlm injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=33a147be-233a-40e8-a55e-e40936e28db0
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
EMGALITY- galcanezumab-gnlm injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0002-1436-01 |
| 2 | 0002-1436-11 |
| 3 | 0002-1436-27 |
| 4 | 0002-1436-61 |
| 5 | 0002-2377-01 |
| 6 | 0002-2377-11 |
| 7 | 0002-2377-27 |
| 8 | 0002-3115-01 |
| 9 | 0002-3115-09 |