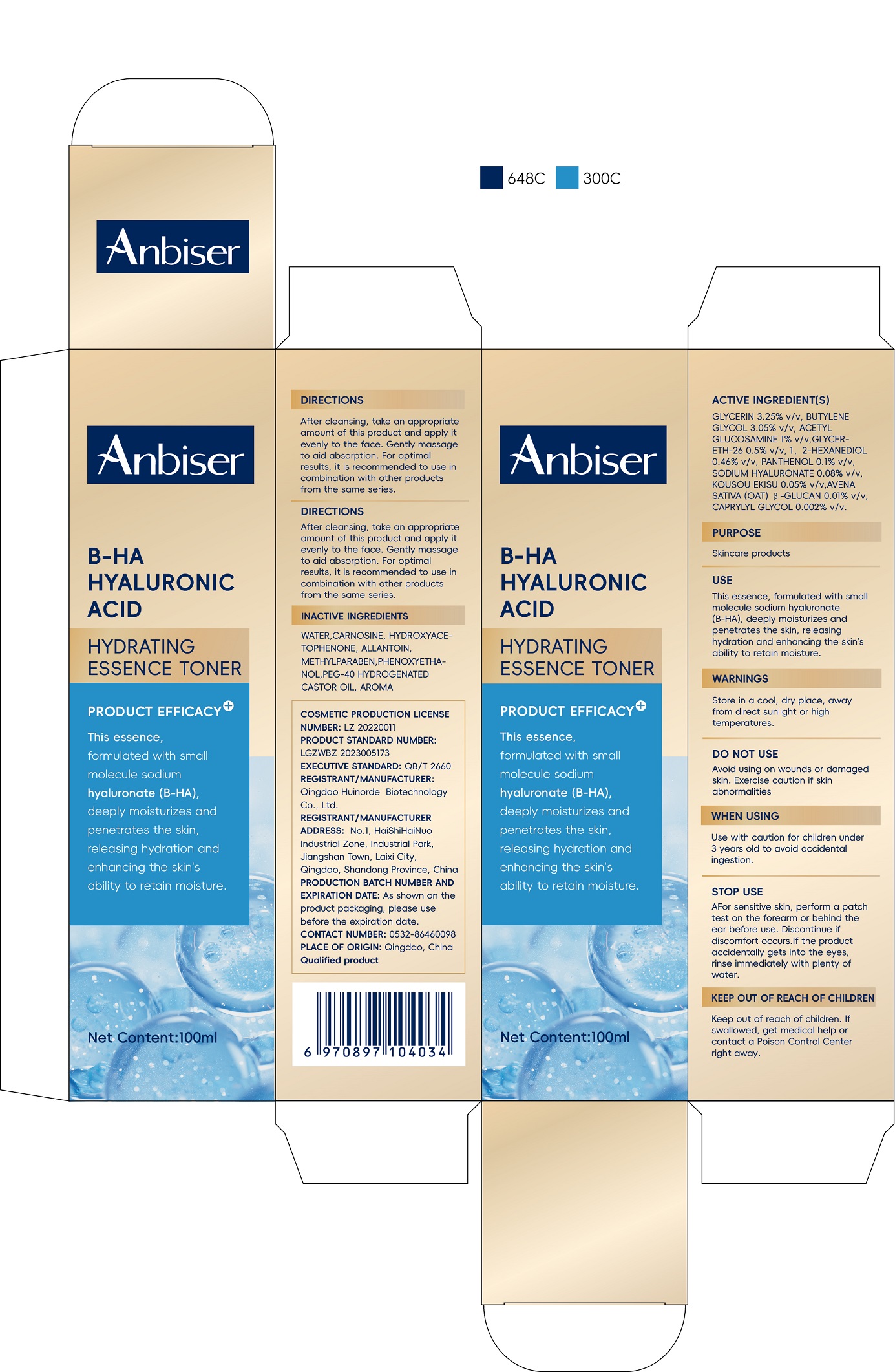
Label: ANBISER B-HA HYALURONIC ACID HYDRATING ESSENCE TONER- glycerin solution
- NDC Code(s): 84951-005-01
- Packager: Qingdao Huinorde Biotechnology Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredient
GLYCERIN 5.25% v/v, CAPRYLIC/CAPRIC TRIGLYCERIDE 4.0765% v/v, SORBITOL 3% v/v, ETHYLHEXYL PALMITATE 2% v/v,BUTYROSPERMUM PARKII (SHEA) BUTTER 0.25% v/v, PANTHENOL 0.2% v/v, ALGAE EXTRACT 0.15% v/v,SODIUM HYALURONATE 0.134% v/v,MACROCYSTIS PYRIFERA (KELP) EXTRACT 0.1% v/v,RED ALGAE EXTRACT 0.05% v/v, BUTYLENE GLYCOL 0.025% v/v, AVENASATIVA(OAT)β-GLUCAN 0.005% v/v, 1,2-HEXANEDIOL 0.005% v/v, CAPRYLYL GLYCOL 0.004% v/v
- Warnings
- INACTIVE INGREDIENTS
- Directions
- USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- DO NOT USE
- WHEN USE
- STOP USE
- STORAGE AND HANDLING
- PACKAGE
-
INGREDIENTS AND APPEARANCE
ANBISER B-HA HYALURONIC ACID HYDRATING ESSENCE TONER
glycerin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84951-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 3.25 g in 100 mL BUTYLENE GLYCOL (UNII: 3XUS85K0RA) (BUTYLENE GLYCOL - UNII:3XUS85K0RA) BUTYLENE GLYCOL 3.05 g in 100 mL N-ACETYLGLUCOSAMINE (UNII: V956696549) (ACETYL GLUCOSAMINE - UNII:V956696549) N-ACETYLGLUCOSAMINE 1 g in 100 mL GLYCERETH-26 (UNII: NNE56F2N14) (GLYCERETH-26 - UNII:NNE56F2N14) GLYCERETH-26 0.5 g in 100 mL 1,2-HEXANEDIOL (UNII: TR046Y3K1G) (1,2-HEXANEDIOL - UNII:TR046Y3K1G) 1,2-HEXANEDIOL 0.46 g in 100 mL PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.1 g in 100 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 0.08 g in 100 mL CYANIDIUM CALDARIUM WHOLE (UNII: NU386GKK3L) (CYANIDIUM CALDARIUM WHOLE - UNII:NU386GKK3L) CYANIDIUM CALDARIUM WHOLE 0.05 g in 100 mL OAT (UNII: Z6J799EAJK) (AVENA SATIVA (OAT) KERNEL EXTRACT - UNII:Z6J799EAJK) OAT 0.01 g in 100 mL CAPRYLYL GLYCOL (UNII: 00YIU5438U) (CAPRYLYL GLYCOL - UNII:00YIU5438U) CAPRYLYL GLYCOL 0.002 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARNOSINE (UNII: 8HO6PVN24W) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ALLANTOIN (UNII: 344S277G0Z) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) DICHROSTACHYS CINEREA WHOLE (UNII: N27D64ME6P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84951-005-01 1 in 1 BOX 11/18/2024 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/18/2024 Labeler - Qingdao Huinorde Biotechnology Co., LTD (541546328) Establishment Name Address ID/FEI Business Operations Qingdao Huinorde Biotechnology Co., LTD 541546328 manufacture(84951-005)