Label: DOXAZOSIN tablet
- NDC Code(s): 0904-5522-61, 0904-5523-61, 0904-5524-61
- Packager: Major Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 60505-0093, 60505-0094, 60505-0095
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXAZOSIN TABLETS safely and effectively. See full prescribing information for DOXAZOSIN TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Benign Prostatic Hyperplasia (BPH) Doxazosin tablets are indicated for the treatment of the signs and symptoms of BPH. 1.2 Hypertension - Doxazosin tablets are indicated for the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Following the initial dose and with each dose increase of doxazosin tablets, monitor blood pressure for at least 6 hours following administration. If doxazosin tablets ...
-
3 DOSAGE FORMS AND STRENGTHSDoxazosin Tablets, USP are available as 1 mg (white to off-white, round, scored tablets, imprinted "APO" on one side and "093" with a partial bisect on the other side), 2 mg (white to off-white ...
-
4 CONTRAINDICATIONSThe use of doxazosin tablets is contraindicated in patients with a hypersensitivity to doxazosin, other quinazolines (e.g., prazosin, terazosin), or any of its components.
-
5 WARNINGS AND PRECAUTIONS5.1 Postural Hypotension - Postural hypotension with or without symptoms (e.g., dizziness) may develop within a few hours following administration of doxazosin tablets. However, infrequently ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 CYP 3A Inhibitors - In vitro studies suggest that doxazosin is a substrate of CYP 3A4. Strong CYP3A inhibitors may increase exposure to doxazosin. Monitor blood pressure and for symptoms of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data with doxazosin tablets in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage ...
-
10 OVERDOSAGEExperience with doxazosin overdosage is limited. Two adolescents, who each intentionally ingested 40 mg doxazosin tablets with diclofenac or acetaminophen, were treated with gastric lavage with ...
-
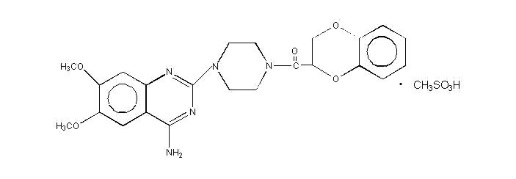
11 DESCRIPTIONDoxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha-adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Benign Prostatic Hyperplasia (BPH) The symptoms associated with benign prostatic hyperplasia (BPH), such as urinary frequency, nocturia, weak stream, hesitancy, and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis and Mutagenesis: Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of ...
-
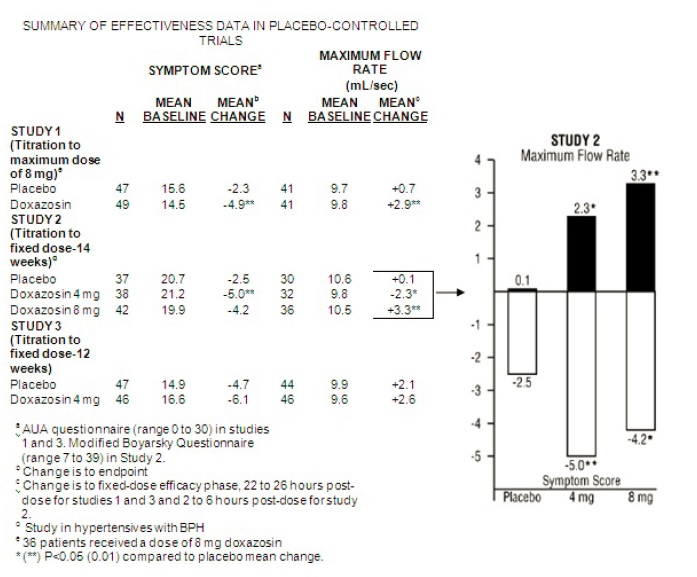
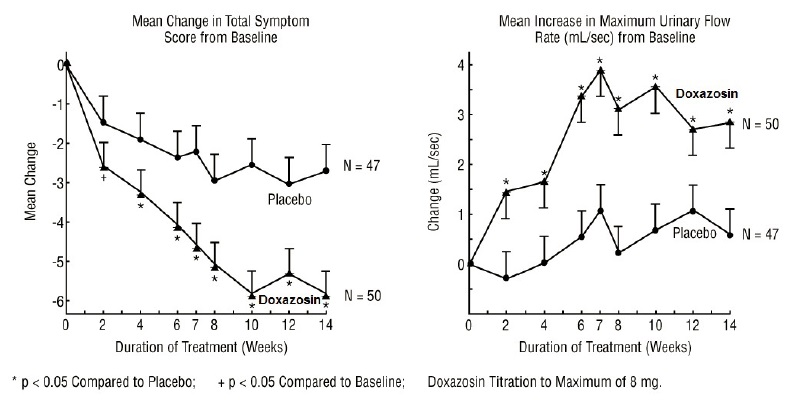
14 CLINICAL STUDIES14.1 Benign Prostatic Hyperplasia (BPH) The efficacy of doxazosin was evaluated extensively in over 900 patients with BPH in double-blind, placebo-controlled trials. Doxazosin tablets treatment ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDoxazosin tablets, USP are available as white to off-white tablets for oral administration. Each tablet contains doxazosin mesylate equivalent to 1 mg, 2 mg, 4 mg or 8 mg of the active ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Postural Hypotension - Advise patients of the possibility of syncopal and orthostatic symptoms, especially at ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Doxazosin Tablets - (dox az' oh sin) What are doxazosin tablets? Doxazosin tablets are a prescription medicine that contains doxazosin mesylate and is called an ...
-
Package/Label Display Panel MAJOR® NDC 0904-5522-61 - Unit Dose - Doxazosin - Tablets, USP - 1 mg* Pharmacist: Dispense with - Patient Information - 100 TABLETS (10 x 10) Rx only
-
Package/Label Display Panel MAJOR® NDC 0904-5523-61 - Unit Dose - Doxazosin - Tablets, USP - 2 mg* Pharmacist: Dispense with - Patient Information - 100 TABLETS (10 x 10) Rx only
-
Package/Label Display Panel MAJOR® NDC 0904-5524-61 - Unit Dose - Doxazosin - Tablets, USP - 4 mg* Pharmacist: Dispense with - Patient Information - 100 TABLETS (10 x 10) Rx only
-
INGREDIENTS AND APPEARANCEProduct Information