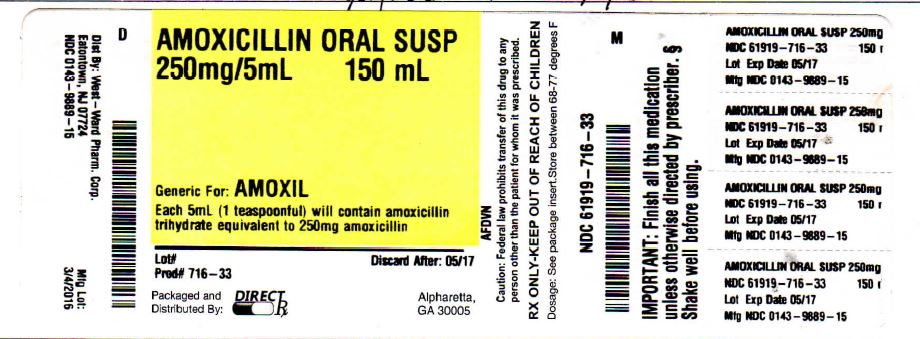
Label: AMOXIL- amoxicillin powder, for suspension
- NDC Code(s): 61919-716-33
- Packager: Direct_Rx
- This is a repackaged label.
- Source NDC Code(s): 0143-9889
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and other antibacterial drugs, Amoxicillin for Oral Suspension, USP should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Amoxicillin for Oral Suspension, USP is indicated in the treatment of infections due to susceptible (ONLY β-lactamase–negative) isolates of the designated bacteria in the conditions listed below:
1.1 Infections of the ear, nose, and throat
- due to Streptococcus species.
(α- and β-hemolytic isolates only), Streptococcus pneumoniae, Staphylococcus spp., or Haemophilus influenzae.
1.2 Infections of the genitourinary tract
- due to Escherichia coli, Proteus mirabilis, or Enterococcus faecalis.
1.3 Infections of the skin and skin structure
- due to Streptococcus spp.
(α- and β-hemolytic isolates only), Staphylococcus spp., or E. coli.
1.4 Infections of the lower respiratory tract
- due to Streptococcus spp.
(α- and β-hemolytic isolates only), S. pneumoniae, Staphylococcus spp., or H. influenzae.
1.5 Gonorrhea, acute uncomplicated (ano-genital and urethral infections in males and females)
- due to Neisseria gonorrhoeae.
Because of high rates of Amoxicillin resistance, Amoxicillin for Oral Suspension, USP is not recommended for empiric treatment of gonorrhea. Amoxicillin for Oral Suspension, USP use should be limited to situations where N. gonorrhoeae isolates are known to be susceptible to Amoxicillin.
1.6 Triple therapy for Helicobacter pylori with clarithromycin and lansoprazole:
Amoxicillin for Oral Suspension, USP, in combination with clarithromycin plus lansoprazole as triple therapy, is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or 1-year history of a duodenal ulcer) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
1.7 Dual therapy for H. pylori with lansoprazole:
Amoxicillin for Oral Suspension, USP, in combination with lansoprazole delayed-release capsules as dual therapy, is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or 1-year history of a duodenal ulcer) who are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected. (See the clarithromycin package insert, MICROBIOLOGY.) Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence. -
DOSAGE AND ADMINSTRATION
2.1 Dosing for Adult and Pediatric Patients > 3 Months of Age
Except for gonorrhea, treatment should be continued for a minimum of 48 to 72 hours beyond the time that the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. It is recommended that there be at least 10 days’ treatment for any infection caused by Streptococcus pyogenes to prevent the occurrence of acute rheumatic fever. In some infections, therapy may be required for several weeks. It may be necessary to continue clinical and/or bacteriological follow-up for several months after cessation of therapy.
Table 1. Dosing Recommendations for Adult and Pediatric Patients > 3 Months of Age Infection Severity a Usual Adult Dose Usual Dose for Children > 3 Monthsb
Ear/Nose/Throat
Skin/ Skin Structure
Genitourinary Tract Mild/
Moderate 500 mg every 12 hours or 250 mg every 8 hours 25 mg/kg/day in divided doses every 12 hours
or
20 mg/kg/day in divided doses every 8 hours
Severe 875 mg every 12 hours or 500 mg every 8 hours 45 mg/kg/day in divided doses every 12 hours
or
40 mg/kg/day in divided doses every 8 hours
Lower Respiratory Tract
Mild/
Moderate or Severe 875 mg every 12 hours or 500 mg every 8 hours 45 mg/kg/day in divided doses every 12 hours
or
40 mg/kg/day in divided doses every 8 hours
Gonorrhea
Acute, uncomplicated ano-genital and urethral infections in males and females 3 grams as single oral dose Prepubertal children:
50 mg/kg Amoxicillin, combined with 25 mg/kg probenecid as a single dose.
Note: since probenecid is contraindicated in children under 2 years, do not use this regimen in children under 2 years of age.a Dosing for infections caused by bacteria that are intermediate in their susceptibility to Amoxicillin should follow the recommendations for severe infections.
b The children’s dosage is intended for individuals whose weight is less than 40 kg. Children weighing 40 kg or more should be dosed according to the adult recommendations.2.2 Dosing in Neonates and Infants Aged ≤ 12 Weeks (≤ 3 Months)
Treatment should be continued for a minimum of 48 to 72 hours beyond the time that the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. It is recommended that there be at least 10 days’ treatment for any infection caused by Streptococcus pyogenes to prevent the occurrence of acute rheumatic fever. Due to incompletely developed renal function affecting elimination of Amoxicillin in this age group, the recommended upper dose of Amoxicillin 30 mg/kg/day divided every 12 hours. There are currently no dosing recommendations for pediatric patients with impaired renal function.
2.3 Dosing for H. pylori Infection
Triple therapy: The recommended adult oral dose is 1 gram Amoxicillin, 500 mg clarithromycin, and 30 mg lansoprazole, all given twice daily (every 12 hours) for 14 days.
Dual therapy: The recommended adult oral dose is 1 gram Amoxicillin and 30 mg lansoprazole, each given three times daily (every 8 hours) for 14 days.
Please refer to clarithromycin and lansoprazole full prescribing information.
2.4 Dosing in Renal Impairment
Patients with impaired renal function do not generally require a reduction in dose unless the impairment is severe.
Severely impaired patients with a glomerular filtration rate of < 30 mL/min. should not receive a 875-mg dose.
Patients with a glomerular filtration rate of 10 to 30 mL/min should receive 500 mg or 250 mg every 12 hours, depending on the severity of the infection.
Patients with a glomerular filtration rate less than 10 mL/min should receive 500 mg or 250 mg every 24 hours, depending on severity of the infection.
Hemodialysis patients should receive 500 mg or 250 mg every 24 hours, depending on severity of the infection. They should receive an additional dose both during and at the end of dialysis.2.5 Directions for Mixing Oral Suspension
Tap bottle until all powder flows freely. Add approximately 1/3 of the total amount of water for reconstitution (see Table 2) and shake vigorously to wet powder. Add remainder of the water and again shake vigorously.
Table 2. Amount of Water for Mixing Oral Suspension Strength Bottle Size Amount of Water
Required for Reconstitution
Oral Suspension 125 mg /5 mL 80 mL 66 mL
100 mL 83 mL
150 mL 125 mL
Oral Suspension 200 mg /5 mL 50 mL 39 mL
75 mL 59 mL
100 mL 78 mL
Oral Suspension 250 mg /5 mL 80 mL 59 mL
100 mL 73 ml
150 mL 110 mL
Oral Suspension 400 mg /5 mL 50 mL 34 mL
75 mL 51 mL
100 mL 68 mLAfter reconstitution, the required amount of suspension should be placed directly on the child’s tongue for swallowing. Alternate means of administration are to add the required amount of suspension to formula, milk, fruit juice, water, ginger ale, or cold drinks. These preparations should then be taken immediately.
NOTE: SHAKE ORAL SUSPENSION WELL BEFORE USING. Keep bottle tightly closed. Any unused portion of the reconstituted suspension must be discarded after 14 days. Refrigeration is preferable, but not required.
- DOSAGE FORMS AND STRENGTHS
- CONTRAINDICATIONS
-
WANRINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy including amoxicillin. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients on oral penicillins. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before initiating therapy with amoxicillin, careful inquiry should be made regarding previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, amoxicillin should be discontinued, and appropriate therapy instituted.
5.2 Severe Cutaneous Adverse Reactions
Amoxicillin may cause severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP). If patients develop skin rash they should be monitored closely, and amoxicillin discontinued if lesions progress.
5.3 Clostridioides difficile-Associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including amoxicillin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Development of Drug-Resistant Bacteria
Prescribing amoxicillin in the absence of a proven or strongly suspected bacterial infection or prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.5 Skin Rash in Patients with Mononucleosis
A high percentage of patients with mononucleosis who receive amoxicillin develop an erythematous skin rash. Thus, amoxicillin should not be administered to patients with mononucleosis.
5.6 Phenylketonurics
Amoxicillin chewable tablets contain aspartame which contains phenylalanine. Each 200 mg chewable tablet contains 1.82 mg phenylalanine; each 400 mg chewable tablet contains 3.64 mg phenylalanine. The oral suspension formulations of amoxicillin do not contain phenylalanine and can be used by phenylketonurics.
-
ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
Anaphylactic reactions [see Warnings and Precautions (5.1)]
Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.2)]
Clostridioides difficile-Associated Diarrhea (CDAD) [see Warnings and Precautions (5.3)]6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions (greater than 1%) observed in clinical trials of amoxicillin oral suspension were diarrhea, rash, vomiting, and nausea.
Triple therapy: The most frequently reported adverse events for patients who received triple therapy (amoxicillin/clarithromycin/ lansoprazole) were diarrhea (7%), headache (6%), and taste perversion (5%).
Dual therapy: The most frequently reported adverse events for patients who received double therapy amoxicillin/lansoprazole were diarrhea (8%) and headache (7%). For more information on adverse reactions with clarithromycin or lansoprazole, refer to the Adverse Reactions section of their package inserts.
6.2 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postmarketing use of penicillins. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to amoxicillin.
Infections and Infestations: Mucocutaneous candidiasis.
Gastrointestinal: Black hairy tongue, and hemorrhagic/pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.3)].
Immune: Hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock), angioedema, serum sickness-like reactions (urticaria or skin rash accompanied by arthritis, arthralgia, myalgia, and frequently fever), hypersensitivity vasculitis [see Warnings and Precautions (5.1)].
Skin and Appendages: Rashes, pruritus, urticaria, erythema multiforme, SJS, TEN, DRESS, AGEP, exfoliative dermatitis [see Warnings and Precautions (5.2)].
Liver: A moderate rise in AST and/or ALT has been noted, but the significance of this finding is unknown. Hepatic dysfunction including cholestatic jaundice, hepatic cholestasis and acute cytolytic hepatitis have been reported.
Renal: Crystalluria has been reported [see Overdosage (10)].
Hemic and Lymphatic Systems: Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Central Nervous System: Reversible hyperactivity, agitation, anxiety, insomnia, confusion, convulsions, behavioral changes, aseptic meningitis, and/or dizziness have been reported.
Miscellaneous: Tooth discoloration (brown, yellow, or gray staining) has been reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
-
DRUG INTERACTIONS
7.1 Probenecid
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of amoxicillin and probenecid may result in increased and prolonged blood levels of amoxicillin.
7.2 Oral Anticoagulants
Abnormal prolongation of prothrombin time (increased international normalized ratio [INR]) has been reported in patients receiving amoxicillin and oral anticoagulants. Appropriate monitoring should be undertaken when anticoagulants are prescribed concurrently. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation.
7.3 Allopurinol
The concurrent administration of allopurinol and amoxicillin increases the incidence of rashes in patients receiving both drugs as compared to patients receiving amoxicillin alone. It is not known whether this potentiation of rashes is due to allopurinol or the hyperuricemia present in these patients.
7.4 Oral Contraceptives
Amoxicillin may affect intestinal flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.
7.5 Other Antibacterials
Chloramphenicol, macrolides, sulfonamides, and tetracyclines may interfere with the bactericidal effects of penicillin. This has been demonstrated in vitro; however, the clinical significance of this interaction is not well documented.
7.6 Effects on Laboratory Tests
High urine concentrations of ampicillin may result in false-positive reactions when testing for the presence of glucose in urine using CLINITEST®, Benedict’s Solution, or Fehling’s Solution. Since this effect may also occur with amoxicillin, it is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as CLINISTIX®) be used.
Following administration of ampicillin or amoxicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted.
-
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Reproduction studies have been performed in mice and rats at doses up to 2000 mg/kg (3 and 6 times the 3 g human dose, based on body surface area). There was no evidence of harm to the fetus due to amoxicillin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, amoxicillin should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Oral ampicillin is poorly absorbed during labor. It is not known whether use of amoxicillin in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood of the necessity for an obstetrical intervention.
8.3 Nursing Mothers
Penicillins have been shown to be excreted in human milk. Amoxicillin use by nursing mothers may lead to sensitization of infants. Caution should be exercised when amoxicillin is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of amoxicillin for the treatment of upper respiratory tract infections, and infections of the genitourinary tract, skin and skin structure and lower respiratory tract have been established in pediatric patients.
The safety and effectiveness of amoxicillin for the treatment of H.Pylori infection have not been established in pediatric patients.
Because of incompletely developed renal function in neonates and young infants, the elimination of amoxicillin may be delayed. Dosing of amoxicillin should be modified in pediatric patients 12 weeks or younger (3 months or younger) [see Dosage and Administration (2.3)].
8.5 Geriatric Use
An analysis of clinical studies of amoxicillin was conducted to determine whether subjects aged 65 and over respond differently from younger subjects. These analyses have not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Dosing in Renal Impairment
Amoxicillin is primarily eliminated by the kidney and dosage adjustment is usually required in patients with severe renal impairment (GFR less than 30 mL/min). See Dosing in Renal Impairment (2.5) for specific recommendations in patients with renal impairment.
-
OVERDOSAGE
In case of overdosage, discontinue amoxicillin, treat symptomatically, and institute supportive measures as required. A prospective study of 51 pediatric patients at a poison-control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin1.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria.
Renal impairment appears to be reversible with cessation of drug administration. High blood levels may occur more readily in patients with impaired renal function because of decreased renal clearance of amoxicillin. Amoxicillin may be removed from circulation by hemodialysis.
-
DESCRIPTION
Amoxicillin for Oral Suspension, is a semisynthetic antibacterial (amoxicillin), an analog of ampicillin, with a broad spectrum of bactericidal activity against many Gram-positive and Gram-negative microorganisms. Chemically, it is (2S,5R,6R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-zabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate. It may be represented structurally as:
[structural formula]
The amoxicillin molecular formula is C16H19N3O5S•3H2O, and the molecular weight is 419.45.
For Oral Suspension: Each 5 mL of reconstituted suspension contains 200 mg or 400 mg of amoxicillin as the trihydrate. Each 5 mL of the 200 mg and 400 mg reconstituted suspension contains 0.1898 mEq (4.3635 mg) of sodium. Inactive ingredients: colloidal silicon dioxide, hypromellose, sodium benzoate, sucrose, trisodium citrate dihydrate, tutti frutti flavor and xanthan gum.
-
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amoxicillin is an antibacterial drug [see Microbiology 12.4].
12.3 Pharmacokinetics
Absorption: Amoxicillin is stable in the presence of gastric acid and is rapidly absorbed after oral administration. The effect of food on the absorption of amoxicillin from the tablets and suspension of Amoxicillin has been partially investigated; 400 mg and 875 mg formulations have been studied only when administered at the start of a light meal.
Orally administered doses of 250 mg and 500 mg amoxicillin capsules result in average peak blood levels 1 to 2 hours after administration in the range of 3.5 mcg/mL to 5.0 mcg/mL and 5.5 mcg/mL to 7.5 mcg/mL, respectively.
Mean amoxicillin pharmacokinetic parameters from an open, two-part, single-dose crossover bioequivalence study in 27 adults comparing 875 mg of amoxicillin with 875 mg of amoxicillin/clavulanate potassium showed that the 875-mg tablet of Amoxicillin produces an AUC0-∞ of 35.4 ± 8.1 mcg•hr/mL and a Cmax of 13.8 ± 4.1 mcg/mL. Dosing was at the start of a light meal following an overnight fast.
Orally administered doses of amoxicillin suspension, 125 mg/5 mL and 250 mg/5 mL, result in average peak blood levels 1 to 2 hours after administration in the range of 1.5 mcg/mL to 3.0 mcg/mL and 3.5 mcg/mL to 5.0 mcg/mL, respectively.
Oral administration of single doses of 400 mg chewable tablets and 400 mg/5 mL suspension of amoxicillin to 24 adult volunteers yielded comparable pharmacokinetic data:
Table 4: Mean Pharmacokinetic Parameters of Amoxicillin (400 mg chewable tablets and 400 mg/5 mL suspension) in Healthy Adults
Dose*
Amoxicillin
AUC0-∞ (mcg•hr/mL)
Amoxicillin (±S.D.)
Cmax (mcg/mL)†
Amoxicillin (±S.D.)
400 mg (5 mL of suspension) 17.1 (3.1) 5.92 (1.62)
400 mg (1 chewable tablet) 17.9 (2.4) 5.18 (1.64)* Administered at the start of a light meal.
† Mean values of 24 normal volunteers. Peak concentrations occurred approximately 1 hour after the dose.Distribution: Amoxicillin diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid, except when meninges are inflamed. In blood serum, amoxicillin is approximately 20% protein-bound. Following a 1-gram dose, and utilizing a special skin window technique to determine levels of the antibiotic, it was noted that therapeutic levels were found in the interstitial fluid.
Metabolism and Excretion: The half-life of amoxicillin is 61.3 minutes. Approximately 60% of an orally administered dose of amoxicillin is excreted in the urine within 6 to 8 hours. Detectable serum levels are observed up to 8 hours after an orally administered dose of amoxicillin. Since most of the amoxicillin is excreted unchanged in the urine, its excretion can be delayed by concurrent administration of probenecid [see Drug Interactions (7.1)].
12.4 Microbiology
Mechanism of Action
Amoxicillin is similar to penicillin in its bactericidal action against susceptible bacteria during the stage of active multiplication. It acts through the inhibition of cell wall biosynthesis that leads to the death of the bacteria.Resistance
Resistance to amoxicillin is mediated primarily through enzymes called beta-lactamases that cleave the beta-lactam ring of amoxicillin, rendering it inactive.Antimicrobial Activity
Amoxicillin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].Gram-Positive Bacteria
Enterococcus faecalis
Staphylococcus spp.
Streptococcus pneumoniae
Streptococcus spp. (alpha and beta-hemolytic)Gram-Negative Bacteria
Escherichia coli
Haemophilus influenzae
Helicobacter pylori
Proteus mirabilisSusceptibility Testing:
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC. -
NON CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of amoxicillin alone have not been conducted; however, the following information is available from tests on a 4:1 mixture of amoxicillin and potassium clavulanate. Amoxicillin and potassium clavulanate was non-mutagenic in the Ames bacterial mutation assay, and the yeast gene conversion assay. Amoxicillin and potassium clavulanate was weakly positive in the mouse lymphoma assay, but the trend toward increased mutation frequencies in this assay occurred at doses that were also associated with decreased cell survival. Amoxicillin and potassium clavulanate was negative in the mouse micronucleus test and in the dominant lethal assay in mice. Potassium clavulanate alone was tested in the Ames bacterial mutation assay and in the mouse micronucleus test and was negative in each of these assays. In a multi-generation reproduction study in rats, no impairment of fertility or other adverse reproductive effects were seen at doses up to 500 mg/kg (approximately 2 times the 3 g human dose based on body surface area).
-
CLINICAL STUDIES
14.1 H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Randomized, double-blind clinical studies performed in the United States in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within 1 year) evaluated the efficacy of lansoprazole in combination with amoxicillin capsules and clarithromycin tablets as triple 14-day therapy, or in combination with amoxicillin capsules as dual 14-day therapy, for the eradication of H. pylori. Based on the results of these studies, the safety and efficacy of 2 different eradication regimens were established: Triple therapy: Amoxicillin 1 gram twice daily/clarithromycin 500 mg twice daily/lansoprazole 30 mg twice daily (see Table 5). Dual therapy: Amoxicillin 1 gram three times daily/lansoprazole 30 mg three times daily (see Table 6). All treatments were for 14 days. H. pylori eradication was defined as 2 negative tests (culture and histology) at 4 to 6 weeks following the end of treatment. Triple therapy was shown to be more effective than all possible dual therapy combinations. Dual therapy was shown to be more effective than both monotherapies. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
Table 5. H. pylori Eradication Rates When Amoxicillin is Administered as Part of a Triple Therapy Regimen
Study
Triple Therapy Triple TherapyEvaluable Analysisa
[95% Confidence Interval]
(number of patients)
Intent-to-Treat Analysisb
[95% Confidence Interval]
(number of patients)
Study 1
92
[80.0 to 97.7]
(n equals 48)
86
[73.3 to 93.5]
(n equals 55)
Study 2
86
[75.7 to 93.6]
(n equals 66)
83
[72.0 to 90.8]
(n equals 70)
a This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy.
b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy.Table 6. H. pylori Eradication Rates When Amoxicillin is Administered as Part of a Dual Therapy Regimen
Study
Dual Therapy Dual TherapyEvaluable Analysisa
[95% Confidence Interval]
(number of patients)
Intent-to-Treat Analysisb
[95% Confidence Interval]
(number of patients)
Study 1
77
[62.5 to 87.2]
(n equals 51)
70
[56.8 to 81.2]
(n equals 60)
Study 2
66
[51.9 to 77.5]
(n equals 58)
61
[48.5 to 72.9]
(n equals 67)
a This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy.
b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy. - STORAGE AND HANDLING
-
HOW SUPPLIED
Amoxicillin for Oral Suspension, USP: Each 5 mL of reconstituted fruity-flavored suspension contains 200 or 400 mg amoxicillin as the trihydrate.
Amoxicillin for Oral Suspension, USP 200 mg/5mL
50 mL Bottle NDC 0143-9886-50
75mL Bottle NDC 0143-9886-75
100mL Bottle NDC 0143-9886-01Amoxicillin for Oral Suspension, USP 400 mg/5mL
50 mL Bottle NDC 0143-9887-50
75mL Bottle NDC 0143-9887-75
100mL Bottle NDC 0143-9887-01 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMOXIL
amoxicillin powder, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-716(NDC:0143-9889) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 250 mg in 5 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM BENZOATE (UNII: OJ245FE5EU) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI (TUTTI FRUTTI) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-716-33 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065322 03/04/2016 Labeler - Direct_Rx (079254320) Registrant - Direct_Rx (079254320) Establishment Name Address ID/FEI Business Operations Direct_Rx 079254320 repack(61919-716)