Label: FILSUVEZ- birch triterpenes gel
- NDC Code(s): 10122-310-01, 10122-310-02
- Packager: Chiesi USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FILSUVEZ safely and effectively. See full prescribing information for FILSUVEZ. FILSUVEZ® (birch triterpenes) topical gel - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFILSUVEZ is indicated for the treatment of wounds associated with dystrophic and junctional epidermolysis bullosa (EB) in adult and pediatric patients 6 months of age and older.
-
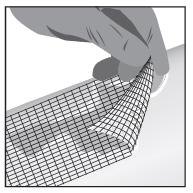
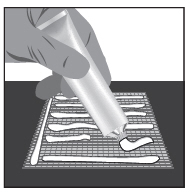
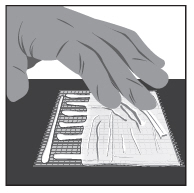
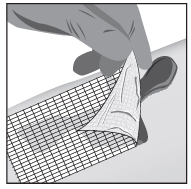
2 DOSAGE AND ADMINISTRATIONWash hands before and after applying FILSUVEZ or wear gloves for application. Apply a 1 mm layer of FILSUVEZ to the affected wound surface only. Do not rub in the gel. Cover the wound with a ...
-
3 DOSAGE FORMS AND STRENGTHSTopical gel: 10% birch triterpenes w/w in a colorless to slightly yellowish, opalescent, non-aqueous gel supplied in 25 mL sterile tubes.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Local hypersensitivity and skin reactions have been reported in patients treated with FILSUVEZ, including urticaria and dermatitis. If signs and symptoms of local ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with use of FILSUVEZ in pregnant women to evaluate for drug-associated risk of major birth defects, miscarriage or adverse maternal ...
-
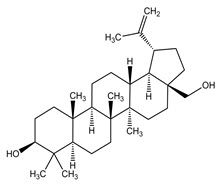
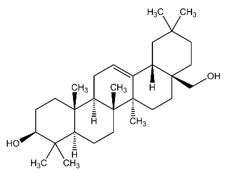
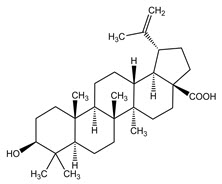
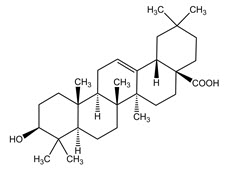
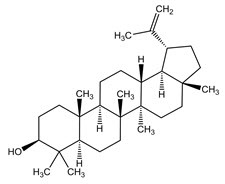
11 DESCRIPTIONFILSUVEZ (birch triterpenes) topical gel is a sterile botanical drug product for topical use and contains birch triterpenes in an oil base. FILSUVEZ is a colorless to slightly yellowish ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of FILSUVEZ in the treatment of wounds associated with epidermolysis bullosa is unknown. 12.2 Pharmacodynamics - Pharmacodynamics of FILSUVEZ ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies have been performed with FILSUVEZ or birch triterpenes. Birch triterpenes were not genotoxic in the in vitro ...
-
14 CLINICAL STUDIESThe efficacy of FILSUVEZ for the treatment of partial-thickness wounds associated with inherited EB was evaluated in a randomized, double-blind, placebo-controlled trial in adults and pediatric ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFILSUVEZ (birch triterpenes) topical gel, 10% (w/w) is a colorless to slightly yellowish, opalescent, non-aqueous gel and is supplied in 25 mL white aluminum tubes containing 23.4 grams of gel per ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Administration Instructions - Advise patients (and/or their caregivers or guardians ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Lichtenheldt GmbH - Pharmazeutische Fabrik - Werk 1 - Industriestr. 7-11 - 23812 Wahlstedt - Germany
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - FILSUVEZ® (fill-sue-vez) (birch triterpenes) topical gel - This Patient Information has been approved by the U.S. Food and Drug AdministrationIssued ...
-
INSTRUCTIONS FOR USEFILSUVEZ® [fill-sue-vez](birch triterpenes)topical gelChiesi - Important information: FILSUVEZ is for use on the skin (topical use) only. Do not use FILSUVEZ in or around your eyes or mucous membranes (for example, the mouth, vagina, or anus). This ...
-
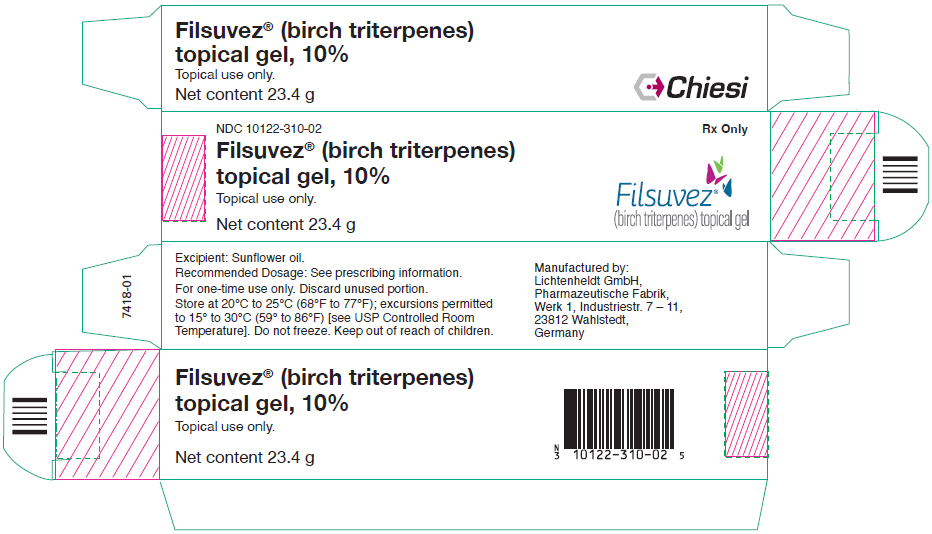
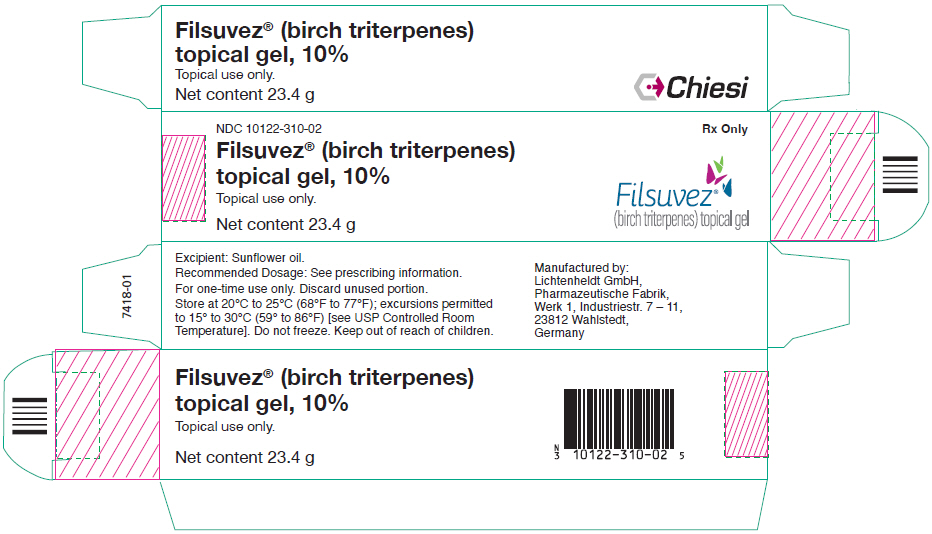
PRINCIPAL DISPLAY PANEL - 23.4 g Tube CartonNDC 10122-310-02 - Rx Only - Filsuvez® (birch triterpenes) topical gel, 10% Topical use only. Net content 23.4 g - Filsuvez® (birch triterpenes) topical gel
-
INGREDIENTS AND APPEARANCEProduct Information