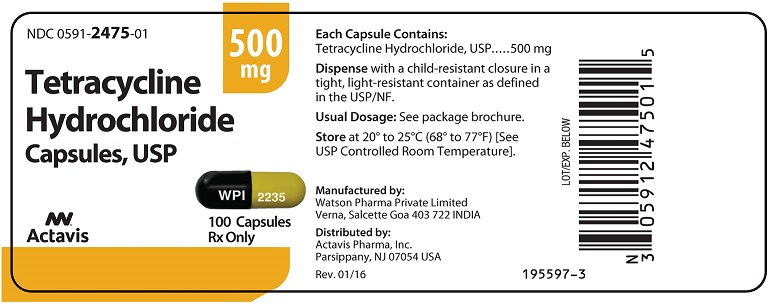
Label: TETRACYCLINE HYDROCHLORIDE capsule
- NDC Code(s): 0591-2474-01, 0591-2475-01
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of tetracycline hydrochloride and other antibacterial drugs, tetracycline hydrochloride should be used only to ...
-
DESCRIPTION
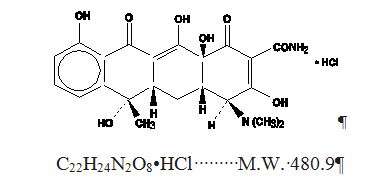
Tetracycline, USP is a yellow, odorless, crystalline powder. Tetracycline, USP is stable in air but exposure to strong sunlight causes it to darken. Its potency is affected in solutions of pH ...
-
CLINICAL
PHARMACOLOGY
Tetracyclines are readily absorbed and are bound to plasma protein in varying degrees. They are concentrated by the liver in the bile and excreted in the urine and feces at high concentrations in ...
-
INDICATIONS AND
USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of tetracycline hydrochloride and other antibacterial drugs, tetracycline hydrochloride should be used only to ...
-
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGS
Tooth Development - The use of drugs of the tetracycline-class during tooth development (last half of pregnancy, infancy and - childhood to the age of 8 years) may cause permanent discoloration of ...
-
PRECAUTIONS
General - As with other antibacterials, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, discontinue antibacterial and institute ...
-
ADVERSE REACTIONS
Gastrointestinal: anorexia, nausea, epigastric distress, vomiting, diarrhea, glossitis, black hairy tongue, dysphagia, enterocolitis, and inflammatory lesions (with Candida overgrowth) in the ...
-
OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Tetracycline is not dialyzable.
-
DOSAGE AND ADMINISTRATION
Adults: Usual daily dose, 1 gram as 500 mg twice a day or 250 mg four times a day. Higher doses such as 500 mg four times a day may be required for severe infections or for those infections which ...
-
HOW SUPPLIED
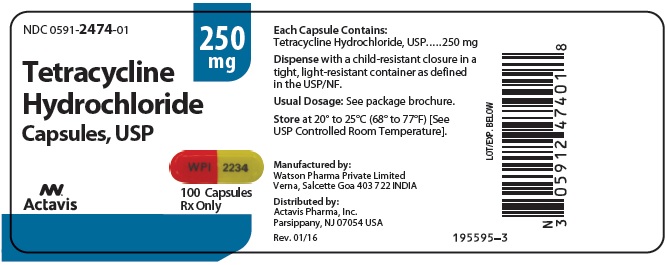
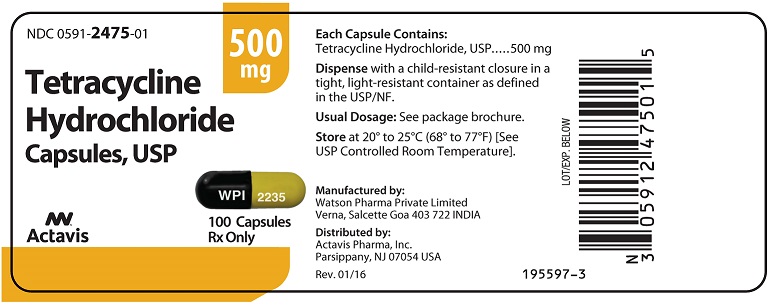
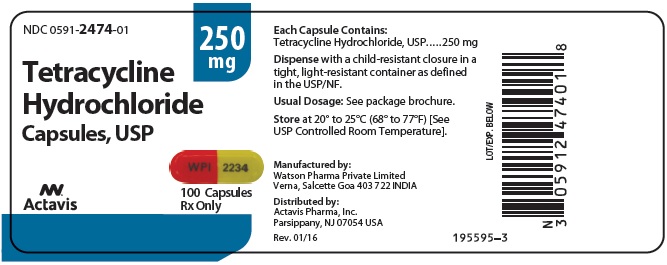
Tetracycline hydrochloride capsules, USP are available as: 250 mg: Capsules with orange opaque cap/yellow opaque body, imprinted with “WPI” on cap and “2234” on body. Available in bottles of 100 ...
-
ANIMAL
PHARMACOLOGY AND ANIMAL TOXICOLOGY
Hyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, minocycline, tetracycline PO4 and ...
-
SPL UNCLASSIFIED SECTIONManufactured In India By: Watson Pharma Private Limited - Verna, Salcette Goa 403 722 India - Manufactured For: Teva Pharmaceuticals - Parsippany, NJ 07054 - Rev. B 4/2025
-
PRINCIPAL DISPLAY PANELNDC 0591-2474-01 - Tetracycline Hydrochloride Capsules, USP - 250 mg - Rx only - 100 Capsules
-
PRINCIPAL DISPLAY PANELNDC 0591-2475-01 - Tetracycline Hydrochloride Capsules, USP - 500 mg - Rx only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information