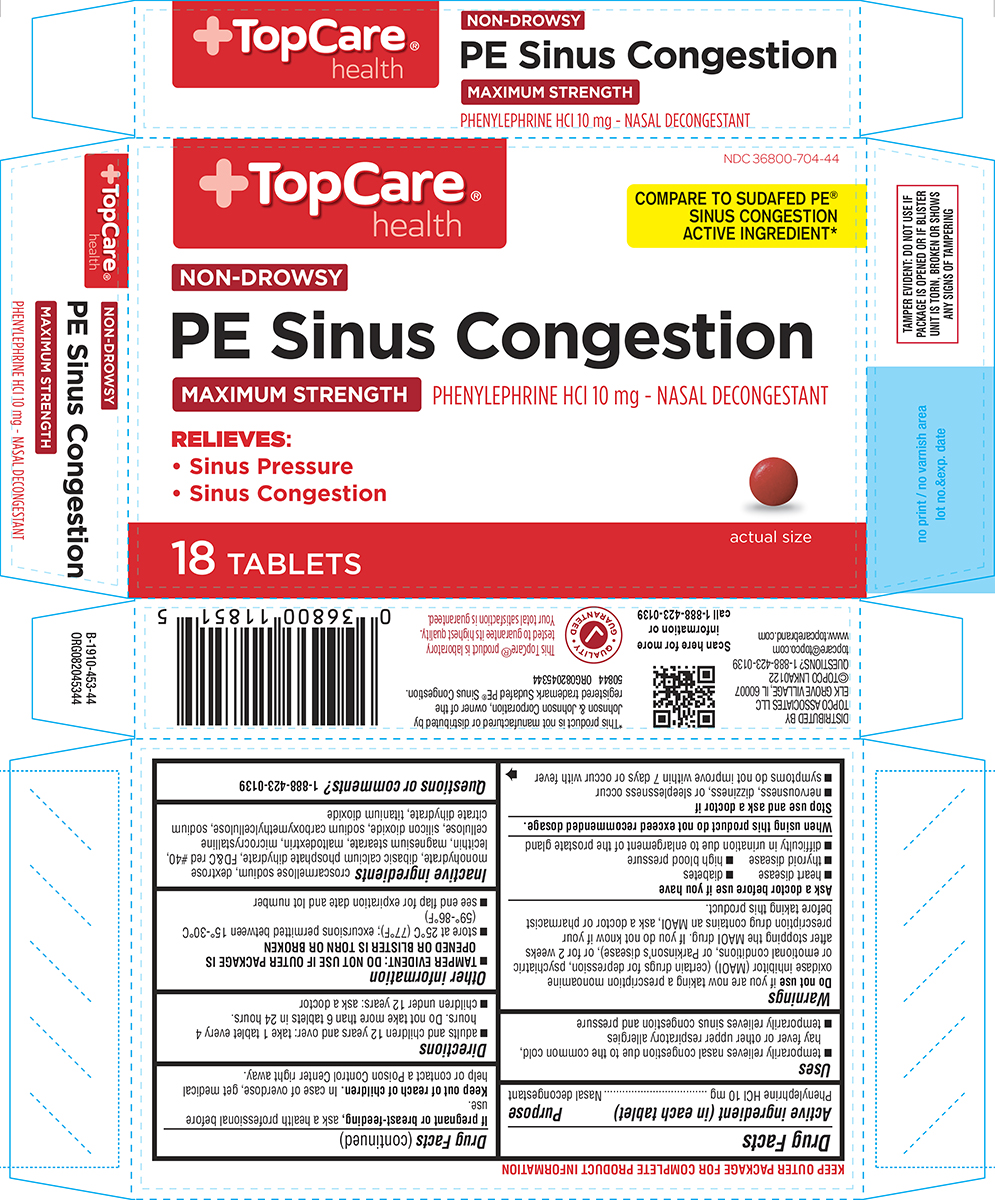
Label: PE SINUS CONGESTION- phenylephrine hcl tablet, film coated
- NDC Code(s): 36800-704-07, 36800-704-29, 36800-704-44
- Packager: Topco Associates, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Phenylephrine HCl 10 mg
-
Purpose
Nasal decongestant
-
Uses
temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies - temporarily relieves sinus congestion and pressure
-
Warnings
Do not use - if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks ...
-
Directions
adults and children 12 years and over: take 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours. children under 12 years: ask a doctor
-
Other information
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN - store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) see end flap for expiration date and ...
-
Inactive ingredients
croscarmellose sodium, dextrose monohydrate, dibasic calcium phosphate dihydrate, FD&C red #40, lecithin, magnesium stearate, maltodextrin, microcrystalline cellulose, silicon dioxide, sodium ...
-
Questions or comments?
1-888-423-0139
-
Principal Display Panel
+TopCare® health - NDC 36800-704-44 - COMPARE TO SUDAFED PE® SINUS CONGESTION - ACTIVE INGREDIENT* NON-DROWSY - PE Sinus Congestion - MAXIMUM STRENGTH - PHENYLEPHRINE HCl 10 mg - NASAL ...
-
INGREDIENTS AND APPEARANCEProduct Information