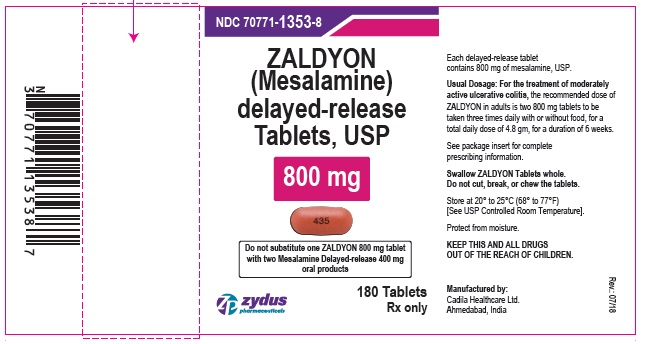
Label: ZALDYON- mesalamine tablet, delayed release
- NDC Code(s): 70771-1353-2, 70771-1353-4, 70771-1353-8
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70771-1353-8 in bottle of 180 tablets - ZALDYON (Mesalamine) delayed-release Tablets USP, 800 mg - Rx only - 180 tablets - ZYDUS
-
INGREDIENTS AND APPEARANCEProduct Information