Label: AMBRISENTAN tablet, film coated
- NDC Code(s): 0591-2405-30, 0591-2406-30
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMBRISENTAN TABLETS safely and effectively. See full prescribing information for AMBRISENTAN TABLETS. AMBRISENTAN tablets, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
Do not administer ambrisentan to a pregnant female because it may cause fetal harm. Ambrisentan is very likely to produce serious birth defects if used by pregnant females, as this effect has been seen consistently when it is administered to animals [see Contraindications (4.1), Warnings and Precautions (5.1), and Use in Specific Populations (8.1)].
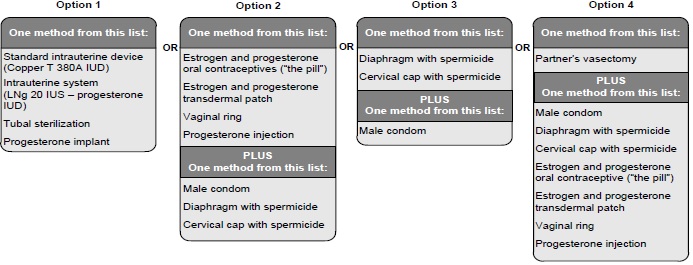
Exclude pregnancy before the initiation of treatment with ambrisentan. Females of reproductive potential must use acceptable methods of contraception during treatment with ambrisentan and for one month after treatment. Obtain monthly pregnancy tests during treatment and 1 month after discontinuation of treatment [see Dosage and Administration (2.2) and Use in Specific Populations (8.3)].
Because of the risk of embryo-fetal toxicity, for all female patients, ambrisentan is only available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Ambrisentan REMS [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGEAmbrisentan tablets are indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1): To improve exercise ability and delay clinical worsening. In combination with ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Dosage - Initiate treatment at 5 mg once daily, with or without tadalafil 20 mg once daily. At 4-week intervals, either the dose of ambrisentan or tadalafil can be increased, as needed ...
-
3 DOSAGE FORMS AND STRENGTHS5 mg and 10 mg film-coated tablets for oral administration - Each 5 mg tablet is white to off-white, capsule shaped, film-coated, and debossed with "5" on one side and "405" on the other side. Each ...
-
4 CONTRAINDICATIONS4.1 Pregnancy - Ambrisentan may cause fetal harm when administered to a pregnant female. Ambrisentan is contraindicated in females who are pregnant. Ambrisentan was consistently shown to have ...
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-fetal Toxicity - Ambrisentan may cause fetal harm when administered during pregnancy and is contraindicated for use in females who are pregnant. In females of reproductive potential ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include: Embryo-fetal Toxicity [see Warnings and Precautions (5.1), Use in Specific Populations ...
-
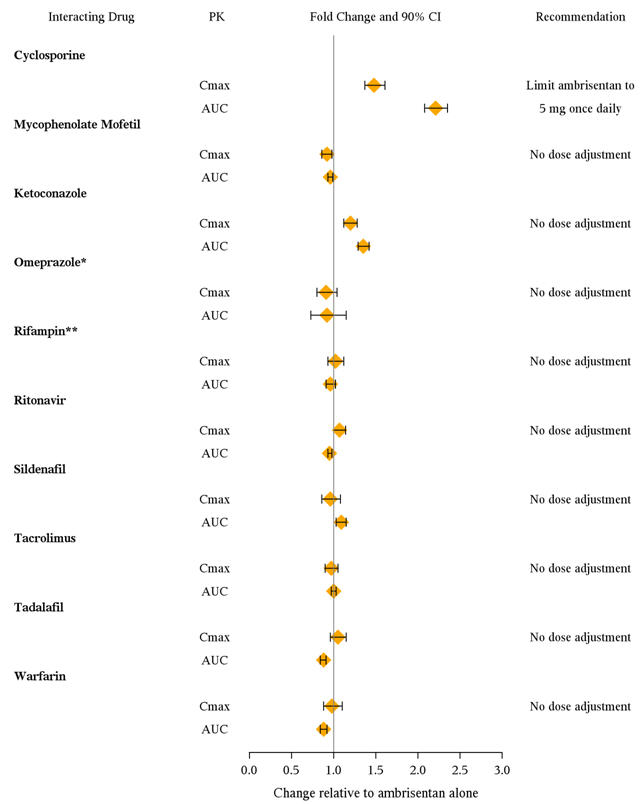
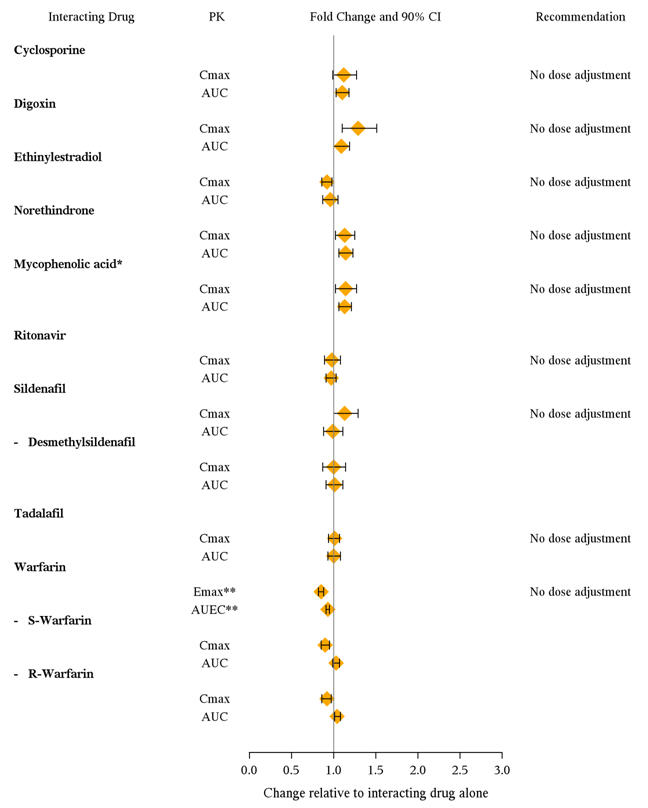
7 DRUG INTERACTIONSMultiple dose coadministration of ambrisentan and cyclosporine resulted in an approximately 2-fold increase in ambrisentan exposure in healthy volunteers; therefore, limit the dose of ambrisentan ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on data from animal reproduction studies, ambrisentan may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy ...
-
10 OVERDOSAGEThere is no experience with overdosage of ambrisentan. The highest single dose of ambrisentan administered to healthy volunteers was 100 mg, and the highest daily dose administered to patients ...
-
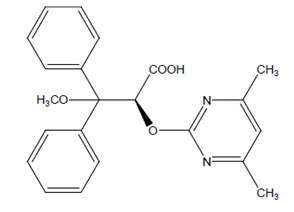
11 DESCRIPTIONAmbrisentan is an endothelin receptor antagonist that is selective for the endothelin type-A (ETA) receptor. The chemical name of ambrisentan is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Endothelin-1 (ET-1) is a potent autocrine and paracrine peptide. Two receptor subtypes, ETA and ETB, mediate the effects of ET-1 in the vascular smooth muscle and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Oral carcinogenicity studies of up to two years duration were conducted at starting doses of 10, 30, and 60 mg/kg/day in rats (8 to 48 ...
-
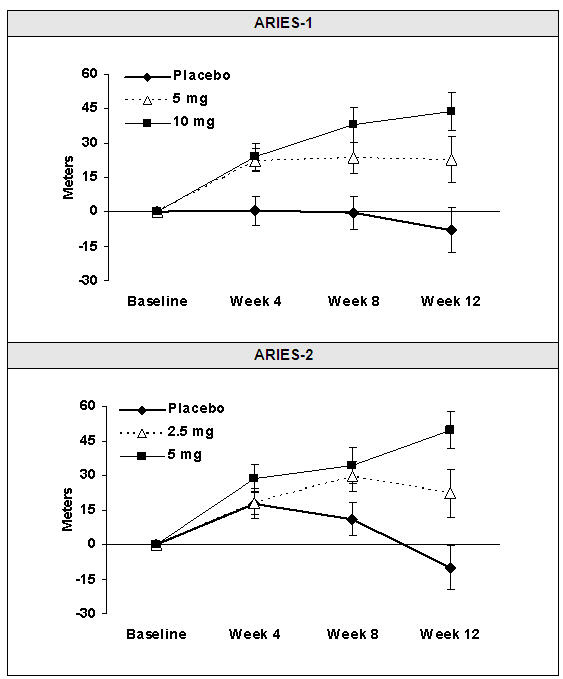
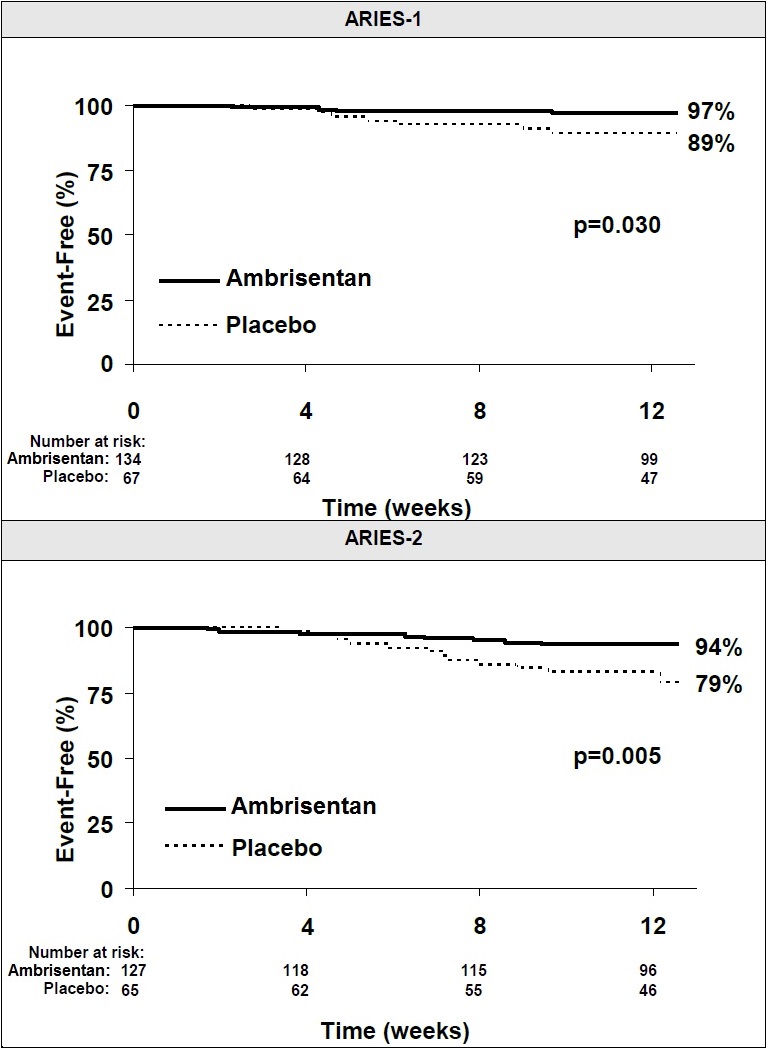
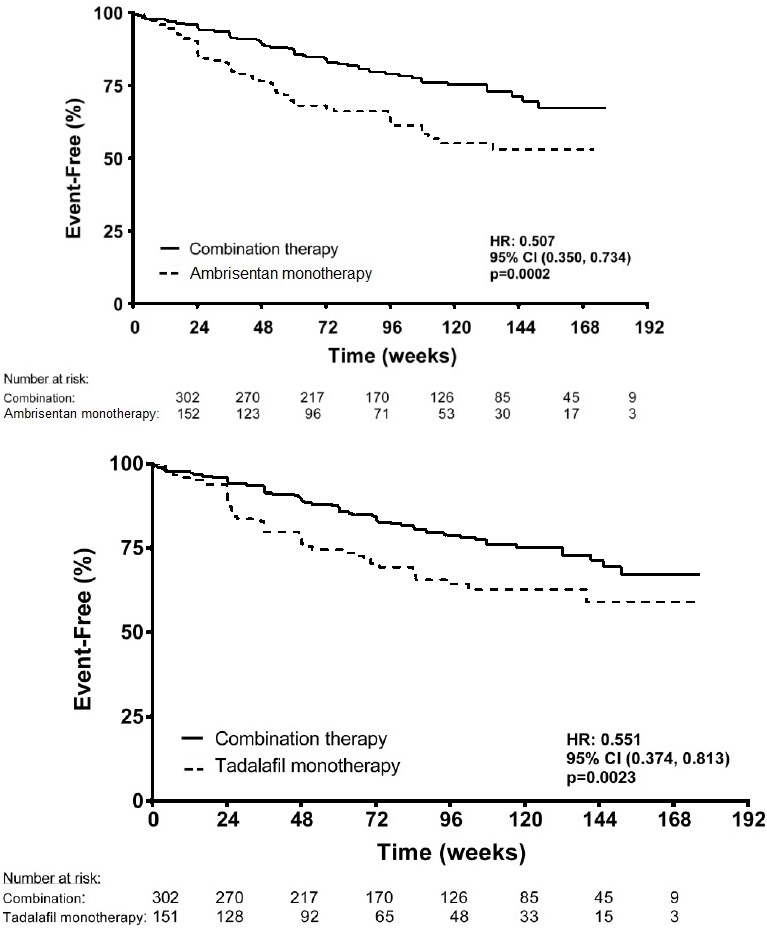
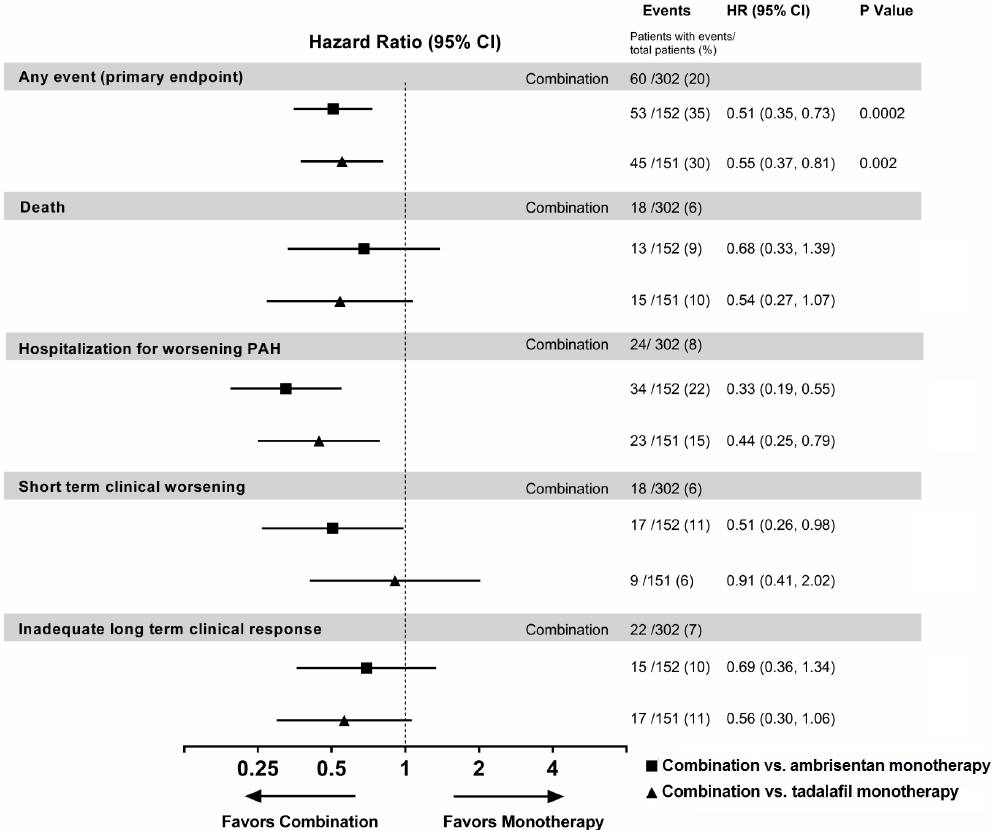
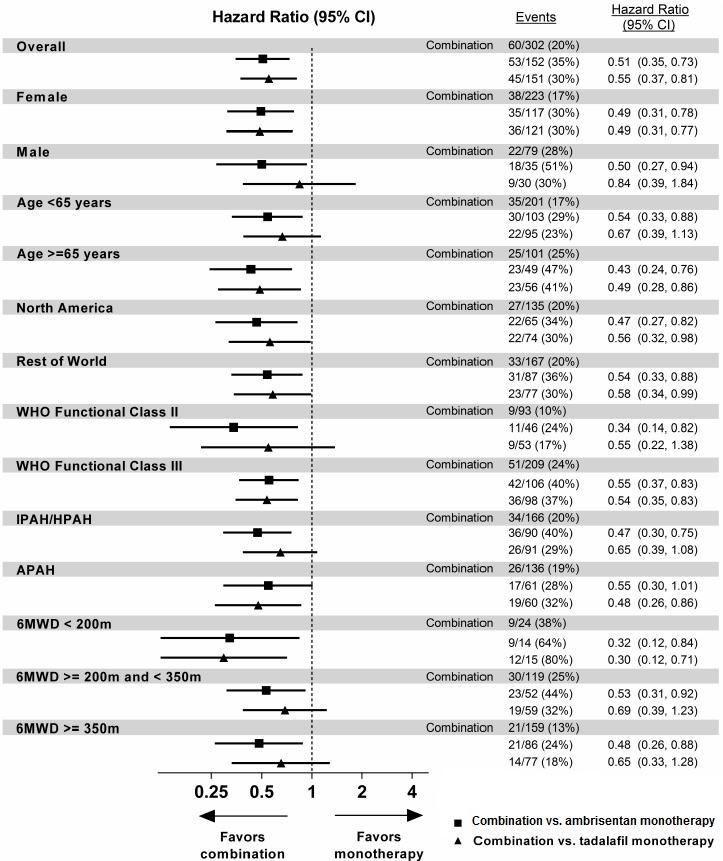
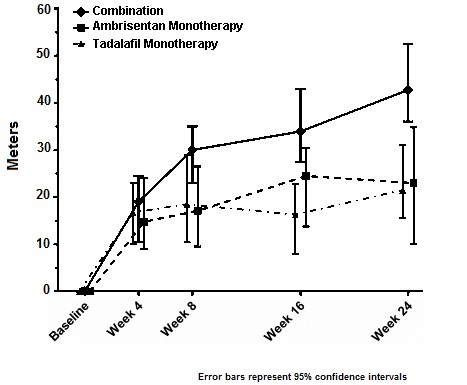
14 CLINICAL STUDIES14.1 Pulmonary Arterial Hypertension (PAH) Two 12-week, randomized, double-blind, placebo-controlled, multicenter studies were conducted in 393 patients with PAH (WHO Group 1). The two studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmbrisentan film-coated tablets are supplied as follows: Tablet Strength - Package - Configuration - NDC No. Description of Tablet; Debossed on Tablet; Size - 5 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Medication Guide). Embryo-fetal Toxicity - Instruct patients on the risk of fetal harm when ambrisentan is used in pregnancy [see ...
-
Medication GuideAmbrisentan (am" bri sen' tan) Tablets - Read this Medication Guide before you start taking ambrisentan tablets and each time you get a refill. There may be new information. This ...
-
PRINCIPAL DISPLAY PANELNDC 0591-2405-30 - Ambrisentan Tablets - 5 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 30 Tablets
-
PRINCIPAL DISPLAY PANELNDC 0591-2406-30 - Ambrisentan Tablets - 10 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 30 Tablets - Teva
-
INGREDIENTS AND APPEARANCEProduct Information