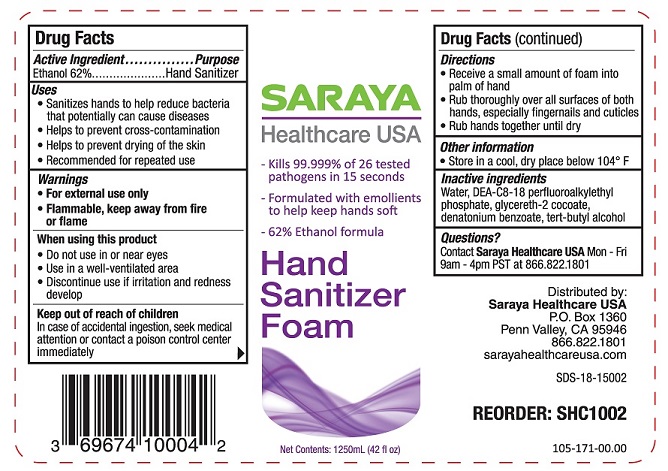
Label: HAND SANITIZER- ethanol liquid
- NDC Code(s): 69674-100-01, 69674-100-04, 69674-100-07
- Packager: Saraya Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SARAYAHealthcare USA
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER
ethanol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69674-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength DIETHANOLAMINE BIS(C8-C18 PERFLUOROALKYLETHYL)PHOSPHATE (UNII: 4J55VM509S) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) WATER (UNII: 059QF0KO0R) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69674-100-01 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/14/2016 2 NDC:69674-100-04 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/14/2016 3 NDC:69674-100-07 45 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/14/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/14/2016 Labeler - Saraya Healthcare (079729980) Establishment Name Address ID/FEI Business Operations Best Sanitizers 627278224 manufacture(69674-100)