Label: IOMERON- iomeprol injection injection, solution
-
NDC Code(s):
76381-725-10,
76381-730-10,
76381-730-20,
76381-735-10, view more76381-735-15, 76381-735-20, 76381-740-10, 76381-740-13, 76381-740-16, 76381-740-20, 76381-930-06, 76381-935-06, 76381-935-09, 76381-940-01, 76381-940-03, 76381-940-06
- Packager: BIPSO GmbH
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
IMPORTANT DRUG INFORMATION
may be found at the following link:
https://imaging.bracco.com/us-en/products/ct-ct-colonography/iomeronSubject: Temporary importation of Iomeron® (iomeprol injection) to address drug shortage issues
Due to the current critical shortages of Omnipaque™ (iohexol injection), Visipaque™ (iodixanol injection), and Ultravist (iopromide injection) in the U.S. market, Bracco Diagnostics Inc. (hereafter “Bracco”) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of iodinated contrast media indicated for intravascular use.
Accordingly, Bracco has initiated temporary importation of Iomeron® (iomeprol injection), an intravascular iodinated contrast medium, into the U.S. market. This product will be used in adult patients by intravenous or intraarterial route of administration only during the shortage. FDA has not approved Iomeron (iomeprol). Iomeron (iomeprol) drug product is manufactured by BIPSO GmbH in Germany and Patheon Italia S.p.A. in Italy. These facilities are also used to manufacture Bracco’s FDA-approved intravascular iodinated contrast medium Isovue (iopamidol injection).
FDA’s regulatory discretion for the importation and distribution of Iomeron (iomeprol) is limited to Bracco during the critical U.S. shortages of Omnipaque, Visipaque, and Ultravist.
Effective immediately, and during this temporary period, Bracco will offer the following presentations of Iomeron (iomeprol) to the U.S. market:
Product Description Iodine Concentration (mg iodine/mL) Package Size Lot Number Label Language NDC Number UK SmPC Iomeron (iomeprol injection) 250 250 56 X 100 mL 2C42685 French 0270-7250-10 Iomeron 300 UK SmPC SD Iomeron (iomeprol injection) 300 300 56 X 100 mL 2C43416
KP1552AFrench 0270-7300-10 Iomeron 300 UK SmPC SD Iomeron (iomeprol injection) 300 300 10 X 200 mL KP2701F English 0270-7300-20 Iomeron 300 UK SmPC SD Iomeron (iomeprol injection) 300 300 6 X 500 mL KP2804A Spanish 0270-9300-06 Iomeron 300 UK SmPC MD Iomeron (iomeprol injection) 350 350 56 X 100 mL LP1566C Russian 0270-7350-10 Iomeron 350 UK SmPC SD Iomeron (iomeprol injection) 350 350 30 X 150 mL 2C42507 French 0270-7350-15 Iomeron 350 UK SmPC SD Iomeron (iomeprol injection) 350 350 10 X 200 mL LP2705A English 0270-7350-20 Iomeron 350 UK SmPC SD Iomeron (iomeprol injection) 350 350 6 X 500 mL LP2807A Spanish 0270-9350-06 Iomeron 350 UK SmPC MD Iomeron (iomeprol injection) 350 350 9 X 500 mL LP2810B English 0270-9350-09 Iomeron 350 UK SmPC MD Iomeron (iomeprol injection) 400 400 56 X 100 mL MP1577A Russian 0270-7400-13 Iomeron 400 UK SmPC SD Iomeron (iomeprol injection) 400 400 10 X 100 mL MP2561C Portuguese 0270-7400-10 Iomeron 400 UK SmPC SD Iomeron (iomeprol injection) 400 400 56 X 100 mL MP2556A Polish 0270-7400-16 Iomeron 400 UK SmPC SD Iomeron (iomeprol injection) 400 400 30 X 200 mL MP2702C Slovenian 0270-7400-20 Iomeron 400 UK SmPC SD Iomeron (iomeprol injection) 400 400 9 X 500 mL MP2807C English 0270-9400-01 Iomeron 400 UK SmPC MD Iomeron (iomeprol injection) 400 400 6 X 500 mL MP2806A English 0270-9400-06 Iomeron 400 UK SmPC MD Iomeron (iomeprol injection) 400 400 9 X 500 mL MP2807A Portuguese 0270-9400-03 Iomeron 400 UK SmPC MD The imported Iomeron (iomeprol) was originally labelled for use in countries outside the United States. The bottle and box labels will display the text used when marketing Iomeron (iomeprol) in those countries. Note that:
- The prescribing information will be provided with each bottle of Iomeron (iomeprol), in the form of the appropriate Summary of Product Characteristics (SmPC) document approved for the U.K., which is written in English, and is representative of all Iomeron (iomeprol) SmPCs for that presentation.
- Copies of the U.K. SmPCs accompany this letter, along with images of the U.K. bottle and box labels that will be imported.
- The Iomeron (iomeprol) U.K. SmPCs are available on-line at: https://imaging.bracco.com/us-en/products/ct-ct-colonography/iomeron
- For those bottles and box labels not in English, English translations of these labels are available on-line at: https://imaging.bracco.com/us-en/products/ct-ct-colonography/iomeron
There are differences among the currently marketed nonionic, low-osmolar iodinated contrast media in their physico-chemical properties, as can be seen in the below table that compares them at the concentration of 300 mg iodine/mL (except for Visipaque, for which the nearest concentration is 320 mg iodine/mL):
Table of Physico-chemical Properties of Iomeron (iomeprol) vs. Comparable U.S. Marketed Products (using a concentration of 300 mg iodine/mL or nearest equivalent) Product Viscosity (CP) Osmolality
(mOsm/kg water)Density pH 20°C 37°C 37°C 37°C Iomeron
(iomeprol injection) 3008.1 4.5 521 1.334 6.5 – 7.2 Iomeron
(iomeprol injection) 3008.8 4.7 616 1.339 6.5 – 7.5 Omnipaque
(iohexol injection) 30011.8 6.3 672 1.349 6.8 – 7.7 Optiray (ioversol injection) 300 8.2 (25°C) 5.5 651 1.352 6.0 – 7.4 Visipaque
(iodixanol injection) 32026.6 11.8 290 1.356 6.8 – 7.7 Ultravist
(iopromide injection) 3009.2 4.9 607 1.322 6.5 – 8.0 Iomeron multi-dose container’s administration:
The 500 mL presentations of Iomeron are multi-dose containers. The U.K. SmPC states that the Iomeron multi-dose bottle stopper should be pierced only once, and that proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium should be used. For those injectors in which the Iomeron container would be directly inserted (i.e., there would be no use of a transfer set), the injector manufacturer’s procedures for insertion should be followed, keeping in mind that the bottle stopper should be pierced only once.
Microbial contamination studies were performed where Iomeron solutions in multi-dose containers were inoculated with micro-organisms. These studies demonstrated that Iomeron solutions are bacteriostatic, with microbial growth not observed over the 10-hour period of the studies. Based upon these studies, when the 500 mL multi-dose container is used to draw up or administer separate doses of Iomeron, any unused product remaining in the bottle after 10 hours from the stopper being pierced must be discarded.
Please see Appendix 1 of this letter for tables showing the differences among the FDA-approved intra-arterial and intravenous indications for Omnipaque (iohexol), Visipaque (iodixanol), Ultravist (iopromide) and Isovue (iopamidol) vs. the intra-arterial and intravenous indications for Iomeron (iomeprol) approved in the U.K.
Iomeron (iomeprol) will be available only by prescription in the U.S. However, the imported lots do not have the statement “Rx only” on their labeling. Please refer to the Iomeron (iomeprol) U.K. SmPC for the product’s full prescribing information. In addition, please note the following comments and recommendations:
- There are differences between indications for Iomeron (iomeprol) approved in the U.K. and approved indications for iodinated contrast media (ICM) in the US. Tables comparing indications for selected FDA approved ICM and Iomeron (iomeprol) are provided in Appendix 1.
- We recommend that imported Iomeron (iomeprol) be administered only by intravenous and intra-arterial routes.
- We recommend that imported Iomeron (iomeprol) be used only in adult patients. Iomeron (iomeprol) adult dosing per the U.K. SmPC is provided in Appendix 2.
- Obtain a history of allergy, hypersensitivity, or hypersensitivity reactions to iodinated contrast agents and always have emergency resuscitation equipment and trained personnel available prior to Iomeron administration. Monitor all patients for hypersensitivity reactions.
- Use the lowest necessary dose of Iomeron (iomeprol) in patients with renal impairment or with congestive heart failure.
- Avoid angiocardiography whenever possible in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
- Thyroid storm has occurred after the intravascular use of iodinated contrast agents in patients with hyperthyroidism, or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of any iodinated contrast agent.
- Administer iodinated contrast agents with extreme caution in patients with known or suspected pheochromocytoma. Inject the minimum amount of contrast necessary, assess the blood pressure throughout the procedure, and have measures for treatment of a hypertensive crisis readily available.
- Severe cutaneous adverse reaction severity may increase and time to onset may decrease with repeat administration of contrast agents; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering Iomeron to patients with a history of a severe cutaneous adverse reaction to Iomeron.
- Stop metformin at the time of, or prior to, Iomeron (Iomeron) administration in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure and reinstitute metformin only after renal function is stable.
- Administration of iodinated contrast agents may interfere with thyroid uptake of radioactive iodine (I-131 and I-123) and decrease therapeutic and diagnostic efficacy in patients with carcinoma of the thyroid. The decrease in efficacy lasts for 6 to 8 weeks.
- Renal toxicity has been reported in a few patients with liver dysfunction who were given an oral cholecystographic agent followed by intravascular iodinated contrast agents. Administration of any intravascular iodinated contrast agent should therefore be postponed in patients who have recently received a cholecystographic contrast agent.
The Iomeron (iomeprol) barcode may not register accurately on U.S. barcode scanning systems. Institutions should manually input the product into their systems and confirm that their systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
To place an order for Iomeron (iomeprol), please contact Bracco Customer Service at 1-877-272-2269 or at Bracco.otc@diag.bracco.com. Hours of operation: Monday-Friday 8:30 AM – 6:00 PM EDT, excluding holidays.
To report adverse events associated with the use of this product, please contact Bracco Drug Safety at 1-800-257-5181, option 1, or at adverse.events@diag.bracco.com.
To report quality problems, or if you have any questions about the information contained in this letter or the use of Iomeron (iomeprol), please contact Bracco Professional Services at 1-800-257-5181, option 2, or at services.professional@diag.bracco.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178)
Alberto Spinazzi, MD
Senior Vice President
Chief Medical and Regulatory Officer
Bracco GroupAttachments:
Iomeron (iomeprol) U.K. SmPCs
Images of Iomeron (iomeprol) U.K. Bottle and Box LabelsAppendix 1. Comparisons of approved uses for iodinated contrast media in shortage and those manufactured by Bracco.
Table of Approved Intra-arterial Uses for Iodinated Contrast Media in Shortage, and for Those Manufactured by Bracco POPULATION/
INDICATIONOMNIPAQUE
(iohexol)
mg iodine/mLVISIPAQUE
(iodixanol)
mg iodine/mLULTRAVIST
(iopromide)
mg iodine/mLISOVUE
(iopamidol)
mg iodine/mLIOMERON*
(iomeprol)
mg iodine/mLADULTS Intra-arterial digital subtraction angiography 140 270, 320 250, 300 Aortography 300, 350 370 350, 400 Angiocardiography 350 320 370 370 300, 350, 400 Visceral arteriography 300, 350 320 370 370 300, 350, 400 Cerebral arteriography 300 320 300 300 250, 300 Peripheral arteriography 300, 350 320 300 300 300, 350, 400 *Approved uses for Iomeron (iomeprol) are from the U.K. Table of Approved Intravenous Uses for Iodinated Contrast Media in Shortage, and for Those Manufactured by Bracco POPULATION/
INDICATIONOMNIPAQUE
(iohexol)
mg iodine/mLVISIPAQUE
(iodixanol)
mg iodine/mLULTRAVIST
(iopromide)
mg iodine/mLISOVUE
(iopamidol)
mg iodine/mLIOMERON*
(iomeprol)
mg iodine/mLADULTS CT head 240, 300, 350 270, 320 300, 370 250, 300 250, 300, 350 CT body 300, 350 270, 320 300, 370 250, 300 250, 300, 350, 400 Intravenous digital subtraction angiography 350 250, 300, 350, 400 Peripheral venography 240, 300 270 200 250, 300, 350 Excretory urography 300, 350 270, 320 300 250, 300, 370 250, 300, 350, 400 *Approved uses for Iomeron (iomeprol) are from the U.K. Appendix 2. Iomeron (iomeprol injection) adult dosing recommendations per U.K. Summary of Product Characteristics.
Table of adult dosing recommendations for Iomeron 250 single dose
(* Repeat as necessary)Venography 10 – 100 mL*
maximum 250 mL
10 – 50 mL upper extremity
50 – 100 mL lower extremityCerebral arteriography 5 – 12 mL* Digital subtraction angiography Intra arterial visceral 2 – 20 mL per artery*
aorta 25-50 mL*
both 250 mL maximumperipheral 5 – 10 mL per artery*
maximum 250 mLIntravenous 30 – 60 mL*
maximum 250 mLComputed tomography brain 50 – 150 mL body 40 – 150 mL
maximum 250 mLUrography intravenous 50 – 150 mL Table of adult dosing recommendations for Iomeron 300 single dose
(* Repeat as necessary)Peripheral arteriography 10 – 90 mL* Venography 10 – 100 mL*
maximum 250 mL
10 – 50 mL upper extremity
50 – 100 mL lower extremityAngiocardiography and left ventriculography 30 – 80 mL
maximum 250 mLCerebral arteriography 5 – 12 mL* Visceral arteriography 5 – 50 mL* or according to type of examination;
maximum 250 mLDigital subtraction angiography Intra arterial visceral 2 – 20 mL per artery*
aorta 25-50 mL*
both 250 mL maximumperipheral 5 – 10 mL per artery*
maximum 250 mLIntravenous 30 – 60 mL*
maximum 250 mLComputed tomography brain 50 – 150 mL body 40 – 150 mL
maximum 250 mLUrography intravenous 50 – 150 mL Table of adult dosing recommendations for Iomeron 300 multidose
(* Repeat as necessary)Computed tomography brain 50 – 150 mL body 40 – 150 mL
maximum 250 mLTable of adult dosing recommendations for Iomeron 350 single dose
(* Repeat as necessary)Peripheral arteriography 10 – 90 mL* Venography 10 – 100 mL*
maximum 250 mL
10 – 50 mL upper extremity
50 – 100 mL lower extremityAortography 50 – 80 mL Angiocardiography and left ventriculography 30 – 80 mL
maximum 250 mLCoronary arteriography 4 – 10 mL per artery* Visceral arteriography 5 – 50 mL* or according to type of examination;
maximum 250 mLIntravenous digital subtraction angiography 30 – 60 mL*
maximum 250 mLComputed tomography brain 50 – 150 mL body 40 – 150 mL
maximum 250 mLUrography intravenous 50 – 150 mL Table of adult dosing recommendations for Iomeron 350 multidose
(* Repeat as necessary)Computed tomography brain 50 – 150 ml body 40 – 150 ml
maximum 250 mLTable of adult dosing recommendations for Iomeron 400 single dose
(* Repeat as necessary)Peripheral arteriography 10 – 90 mL* Aortography 50 – 80 mL Angiocardiography and left ventriculography 30 – 80 mL
maximum 250 mLCoronary arteriography 4 – 10 mL per artery* Visceral arteriography 5 – 50 mL* or according to type of examination Intravenous digital subtraction angiography 30 – 60 mL*
maximum 250 mLComputed tomography of the body 40 – 150 mL
maximum 250 mLUrography intravenous 50 – 150 mL Table of adult dosing recommendations for Iomeron 400 multidose
(* Repeat as necessary)Computed tomography of the body 40 – 150 mL
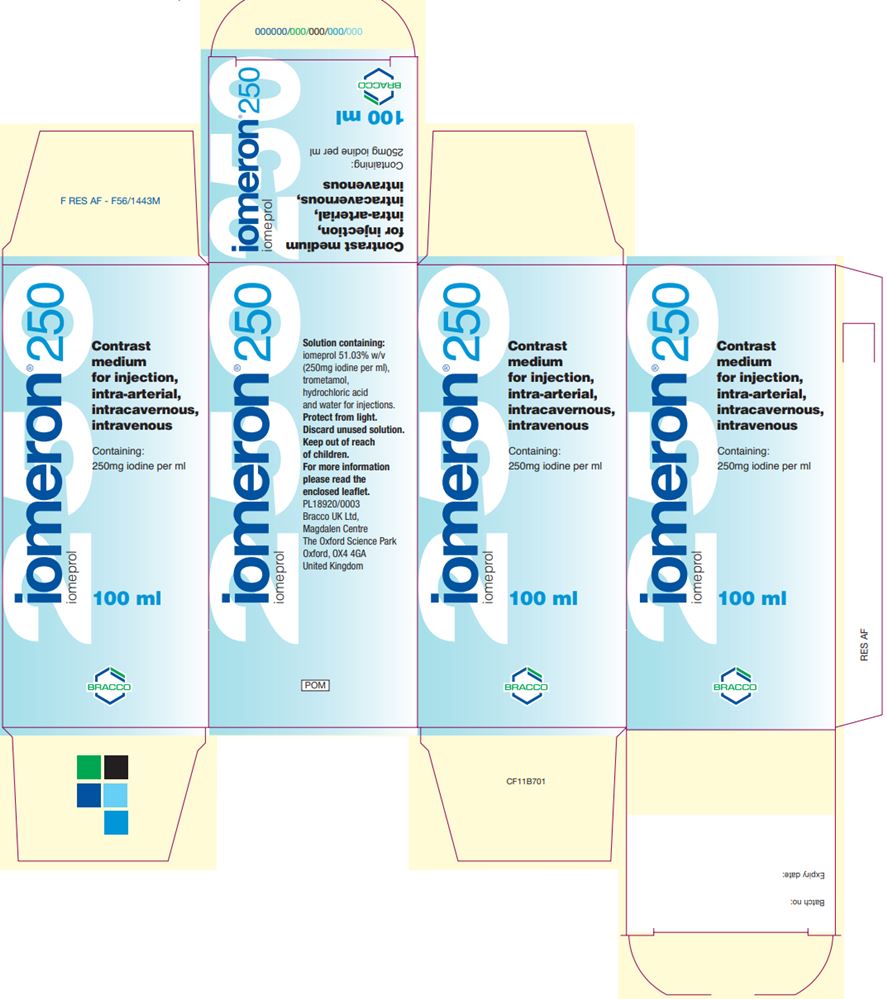
maximum 250 mLUK Iomeron Vial and Carton Labels
Product Description Iodine Concentration (mg iodine/mL) Fill Volume Vial Label Carton Label Iomeron (iomeprol injection) 250 250 100 mL 
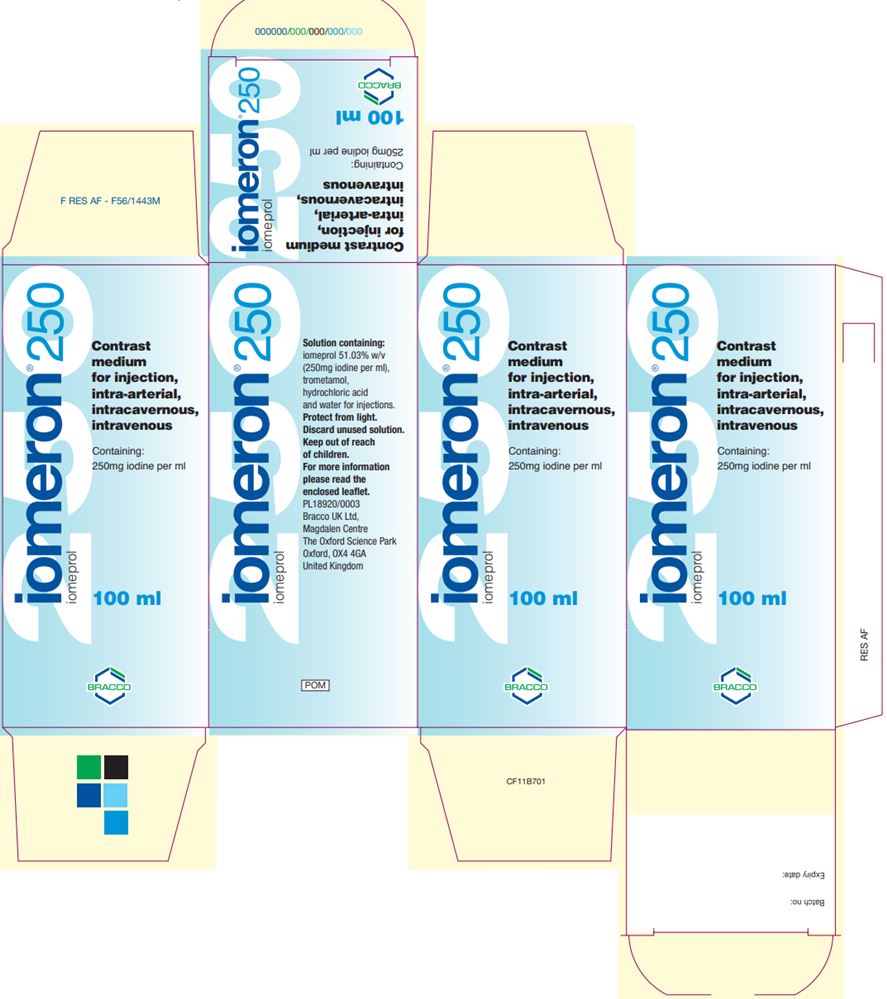
Iomeron (iomeprol injection) 300 300 100 mL 

Iomeron (iomeprol injection) 300 300 200 mL 

Iomeron (iomeprol injection) 300 300 500 mL 

Iomeron (iomeprol injection) 350 350 100 mL 

Iomeron (iomeprol injection) 350 350 200 mL 

Iomeron (iomeprol injection) 350 350 500 mL 

Iomeron (iomeprol injection) 400 400 100 mL 
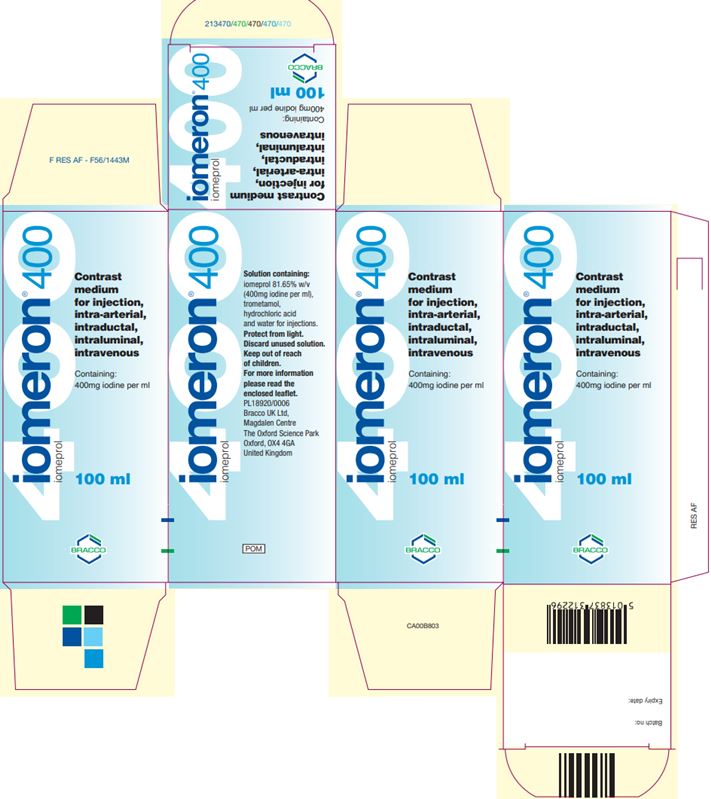
Iomeron (iomeprol injection) 400 400 200 mL 

Iomeron (iomeprol injection) 400 400 500 mL 

-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 250, solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 51.03% w/v of iomeprol equivalent to 25% iodine or 250mg iodine/ml.
For the full list of excipients, see section 6.1.
X-ray contrast medium used for:
- venography
- cerebral arteriography
- digital subtraction angiography
- computed tomography enhancement
- urography
- cavernosography
- myelography
4.2 Posology and method of administration
* Repeat as necessary
* * According to body size and agevenography adults 10 - 100ml* max 250ml 10 - 50ml upper extremity 50 - 100 lower extremity cerebral arteriography adults 5 - 12ml* children 3 - 7ml or * * digital subtraction angiography Intra arterial visceral adults 2 - 20ml per artery* aorta 25-50ml* both 250ml max peripheral adults 5 - 10ml per artery* max 250ml intravenous adults 30 - 60ml* max 250ml computed tomography brain adults 50 - 150 children * * body adults 40 - 150ml max 250ml children * * urography adults 50 - 150ml intravenous neonates 3 - 4.8ml/kg babies 2.5 - 4ml/kg children 1 - 2.5ml/kg or * cavernosography adults 40 - 250ml myelography adults 12 - 18ml by lumbar injection In elderly patients the lowest effective dose should be used.
Unless otherwise instructed by the doctor, a normal diet may be maintained on the day of the examination.
In myelography, lower doses may be used for lumbar or thoracic studies and higher doses for cervical or total columnar studies. Regardless of the nature of the myelographic study, Iomeron should be injected slowly over 1-2 minutes.
The X ray can be taken up to 60 minutes following injection. Post myelographic CT of the spinal column should be delayed for approximately four hours to allow dilution and clearance of excessive contrast.
Hypersensitivity to the active substance or any of the excipients.
Intrathecal concomitant administration of corticosteroids with contrast media is contraindicated.4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
Hydration
Patients must be well hydrated, and any relevant abnormalities of fluid or electrolyte balance should be corrected prior to and following contrast media injection. Especially patients with diabetes mellitus, polyuria, oligouria, hyperuricaemia, infants, small children, and elderly patients, should not be exposed to dehydration. Also patients with severely compromised hepatic and renal impairment are more at risk. Caution should be exercised in hydrating patients with underlying conditions that may be worsened by fluid overload, including congestive heart failure.Rehydration prior to use of iomeprol is recommended in patients with sickle cell disease.
Special population
Hypersensitivity to iodinated contrast media, allergic predispositionA positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:
Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighted against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in severe cardiac disease particularly heart failure and coronary artery disease. Reactions may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias. In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased. In these cases the risks associated with the catheterization procedure are increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease e.g. stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of the contrast medium into cerebral tissue possibly leading to CNS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intra-arterial administration. Premedication with an alpha and beta receptor blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal impairment
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children: Infants up to 1 year, especially the new-born, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates 7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.
Elderly: There is special risk of reactions involving the circulatory system such that myocardial ischaemia, major arrhythmias and extrasystoles are more likely to occur. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.
Precautions for dedicated exams
Angiography
Non ionic contrast media have less antiocoagulant activity in vitro than ionic media. Meticulous attention should therefore be paid to angiographic technique. Non ionic media should not be allowed to remain in contact with blood in a syringe, and intravascular catheters should be flushed frequently to minimise the risk of clotting which, rarely, has led to serious thromboembolic complications.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
Myelography
Following intrathecal use, the patient should rest with the head and the chest elevated for 1 hour and be kept well hydrated. Thereafter, he/she may ambulate carefully, but bending down must be avoided. If remaining in bed, the head and chest should be kept elevated for 6 hours. Patients, suspected of having a lower seizure threshold should be observed during this period.Venography
Special care is required when venography is performed in patients with thrombosis, phlebitis, severe ischaemic disease, local infection or a totally obstructed artero-venous system.4.5 Interaction with other medicinal products and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenotiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
Epidural and intrathecal corticosteroids should never be concurrently administered when iodinated contrast media are used, because corticosteroids may promote and affect the signs and symptoms of arachnoiditis (see section 4.3 - Contraindications).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative. Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible risk.Breastfeeding
No human data exist concerning the excretion of iomeprol in breast milk. Animal studies have demonstrated that the excretion of iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.Stopping breastfeeding is unnecessary.
4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
After intrathecal administration, it is recommended that the patient should wait 24 hours before driving or operating machinery.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
4.8.1 Intravascular administration
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia, Haemolytic anaemia Immune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
Tachycardia
ExtrasystolesCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalCoronary artery thrombosis and coronary artery embolism have been reported as a complication of coronary catheterization procedures.
Vasospasm and consequent ischaemia have been observed during intra-arterial injections of contrast medium, in particular after coronary and cerebral angiography often procedurally related and possibly triggered by the tip of the catheter or excess catheter pressure.
As with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
4.8.2 Intrathecal administration
Adults patients involved in clinical trials with intrathecal administration of Iomeprol were 388.
The most frequently reported adverse reactions following intrathecal administration of Iomeprol are headache, dizziness, nausea, vomiting and back pain. These reactions are usually mild to moderate and transient in nature. Rarely, headache may persist for days. Most side effects occur some hours (3 to 6 hours) after the procedure, due to the distribution of the contrast medium in the CSF circulation from the site of administration to the intravascular space (see section 5.2: Pharmacokinetic properties). Most reactions usually occur within 24 hours after injection.
* Since the reactions were not observed during clinical trials with 388 patients, best estimate is that their relative occurrence is uncommon (≥ 1/1000 to <1/100.
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise application site pain, injection site discomfort, injection site pain and injection site warmth.System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Very common
(≥1/10)Common
(≥1/100 to <1/10)Uncommon
(≥1/1000 to <1/100)Frequency unknown* Immune system disorders Anaphylactoid reaction Nervous system disorders Headache Dizziness Hypoaesthesia
Paraesthesia
Paraparesis
Loss of consciousness
SomnolenceEpilepsy Vascular disorders Hypertension Hypotension
FlushingGastrointestinal disorders Nausea
VomitingSkin and subcutaneous tissue disorders Hyperhidrosis Pruritus Rash Musculoskeletal and connective tissue disorder Back pain
Pain in extremityMusculoskeletal stiffness
Neck painGeneral disorders and administration site conditions Injection site reaction** Feeling hot
PyrexiaNo adverse reactions were reported after intrathecal administration of Iomeprol both in clinical trials and in the post-marketing surveillance.
4.8.3 Administration to body cavities
After injection of an iodinated contrast media in body cavities, contrast media are slowly absorbed from the area of administration into the systemic circulation and subsequently cleared by renal elimination.
Blood amylase increased is common following ERCP. Very rare cases of pancreatitis have been described.
The reactions reported in cases of arthrography and fistulography usually represent irritative manifestations superimposed on pre-existing conditions of tissue inflammation.
Hypersensitivity reactions are rare, generally mild and in the form of skin reactions. However, the possibility of severe anaphylactoid reactions cannot be excluded.
As with other iodinated contrast media, pelvic pain and malaise may occur after hysterosalpingography.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary/ intrathecal contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Following intrathecal administration to animals, iomeprol is completely cleared from the CSF and passes into the plasma compartment.
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of containers
Colourless Type I or Type II glass bottles with rubber/aluminium cap.
Quantities of 20, 30, 50, 75, 100, 150, 200 or 250 ml of solution.6.6 Special precautions for disposal and other handling
Bottles containing contrast media solution are not intended for the withdrawal of multiple doses. The rubber stopper should never be pierced more than once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present.The contrast medium should not be drawn into the syringe until immediately before use. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Sterile techniques must be used with any spinal puncture or intravascular injection, and with catheters and guidewires. If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
It is desirable that solutions of contrast media for intravascular and intrathecal use should be at body temperature when injected.
Any residue of contrast medium in the syringe must be discarded. Solutions not used in one examination session or waste material, such as the connecting tubes, should be disposed in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
11 December 1992 / 29 December 1998
10. DATE OF REVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 300, solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 61.24% w/v of iomeprol equivalent to 30% iodine or 300mg iodine/ml.
For the full list of excipients, see section 6.1.
X-ray contrast medium used for:
- peripheral arteriography
- venography
- angiocardiography and left ventriculography
- cerebral arteriography
- visceral arteriography
- digital subtraction angiography
- computed tomography enhancement
- urography
- ERCP
- dacryocystography
- sialography
- fistulography
- galactography
- myelography
4.2 Posology and method of administration
* Repeat as necessary
* * According to body size and ageperipheral arteriography adults
children10 - 90ml *
* *venography adults 10 - 100ml*
max 250ml
10 - 50ml upper extremity
50 - 100 lower extremityangiocardiography and left ventriculography adults 30 - 80ml max 250ml children * * cerebral arteriography adults 5 - 12ml* children 3 - 7ml or * * visceral arteriography adults 5 - 50ml* or according to type of examination;
max 250mlchildren * * digital subtraction angiography Intra arterial visceral adults 2 - 20ml per artery*
aorta 25-50ml*
both 250ml maxperipheral adults 5 - 10ml per artery*
max 250mlintravenous adults 30 - 60ml*
max 250mlcomputed tomography brain adults 50 - 150ml children * * body adults 40 - 150ml
max 250mlchildren * * urography intravenous adults 50 - 150ml neonates 3 - 4.8ml/kg babies 2.5 - 4ml children 1 - 2.5ml/kg or * arthrography adults 1 - 10ml ERCP adults 12 - 30ml dacryocystography adults 3 - 8ml sialography adults 1 - 3ml fistulography adults 1 - 50ml galactography adults 0.2 - 1.5ml myelography adults 10 - 15ml by lumbar injection In elderly patients the lowest effective dose should be used.
Unless otherwise instructed by the doctor, a normal diet may be maintained on the day of the examination.
In myelography, lower doses may be used for lumbar or thoracic studies and higher doses for cervical or total columnar studies. Regardless of the nature of the myelographic study, Iomeron should be injected slowly over 1-2 minutes.
The X ray can be taken up to 60 minutes following injection. Post myelographic CT of the spinal column should be delayed for approximately four hours to allow dilution and clearance of excessive contrast.
Hypersensitivity to the active substance or any of the excipients.
Intrathecal concomitant administration of corticosteroids with contrast media is contraindicated.4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
Hydration
Patients must be well hydrated, and any relevant abnormalities of fluid or electrolyte balance should be corrected prior to and following contrast media injection. Especially patients with diabetes mellitus, polyuria, oligouria, hyperuricaemia, infants, small children, and elderly patients, should not be exposed to dehydration. Also patients with severely compromised hepatic and renal impairment are more at risk. Caution should be exercised in hydrating patients with underlying conditions that may be worsened by fluid overload, including congestive heart failure.Rehydration prior to use of iomeprol is recommended in patients with sickle cell disease.
Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighted against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in severe cardiac disease particularly heart failure and coronary artery disease. Reactions may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias.In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased. In these cases the risks associated with the catheterization procedure are increased.
The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of the contrast medium into cerebral tissue possibly leading to CMS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intra-arterial administration. Premedication with an alpha and beta receptor blocker is recommended in these patients.
Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal impairment
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters, in particular before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children:
Infants up to 1 year, especially the new-born, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.Transient hypothyroidism may occur in neonates when the mother or the neonate has received an iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates 7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.
Elderly:
There is special risk of reactions involving the circulatory system such that myocardial ischaemia, major arrhythmias and extrasystoles are more likely to occur. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.Precautions for dedicated exams
Angiography
Non ionic contrast media have less antiocoagulant activity in vitro than ionic media. Meticulous attention should therefore be paid to angiographic technique. Non ionic media should not be allowed to remain in contact with blood in a syringe, and intravascular catheters should be flushed frequently to minimise the risk of clotting which, rarely, has led to serious thromboembolic complications.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
Myelography
Following intrathecal use, the patient should rest with the head and the chest elevated for 1 hour and be kept well hydrated. Thereafter, he/she may ambulate carefully, but bending down must be avoided. If remaining in bed, the head and chest should be kept elevated for 6 hours. Patients, suspected of having a lower seizure threshold should be observed during this period.Venography
Special care is required when venography is performed in patients with thrombosis, phlebitis, severe ischaemic disease, local infection or a totally obstructed artero-venous system.4.5 Interaction with other medicaments and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenotiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
Epidural and intrathecal corticosteroids should never be concurrently administered when iodinated contrast media are used, because corticosteroids may promote and affect the signs and symptoms of arachnoiditis (see section 4.3 - Contraindications).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.
Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible risk.Breastfeeding
No human data exist concerning the excretion of iomeprol in breast milk. Animal studies have demonstrated that the excretion of iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.Stopping breastfeeding is unnecessary.
4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
After intrathecal administration, it is recommended that the patient should wait 24 hours before driving or operating machinery.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
4.8.1 Intravascular administration
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia, Haemolytic anaemia Immune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
Tachycardia
ExtrasystolesCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalCoronary artery thrombosis and coronary artery embolism have been reported as a complication of coronary catheterization procedures.
Vasospasm and consequent ischaemia have been observed during intra-arterial injections of contrast medium, in particular after coronary and cerebral angiography often procedurally related and possibly triggered by the tip of the catheter or excess catheter pressure.
As with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
4.8.2 Intrathecal administration
Adults patients involved in clinical trials with intrathecal administration of Iomeprol were 388.
The most frequently reported adverse reactions following intrathecal administration of Iomeprol are headache, dizziness, nausea, vomiting and back pain. These reactions are usually mild to moderate and transient in nature. Rarely, headache may persist for days. Most side effects occur some hours (3 to 6 hours) after the procedure, due to the distribution of the contrast medium in the CSF circulation from the site of administration to the intravascular space (see section 5.2: Pharmacokinetic properties). Most reactions usually occur within 24 hours after injection.
* Since the reactions were not observed during clinical trials with 388 patients, best estimate is that their relative occurrence is uncommon (≥ 1/1000 to <1/100.
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise application site pain, injection site discomfort, injection site pain and injection site warmth.System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Very common
(≥1/10)Common
(≥1/100 to <1/10)Uncommon
(≥1/1000 to <1/100)Frequency unknown* Immune system disorders Anaphylactoid reaction Nervous system disorders Headache Dizziness Hypoaesthesia
Paraesthesia
Paraparesis
Loss of consciousness
SomnolenceEpilepsy Vascular disorders Hypertension Hypotension
FlushingGastrointestinal disorders Nausea
VomitingSkin and subcutaneous tissue disorders Hyperhidrosis Pruritus Rash Musculoskeletal and connective tissue disorder Back pain
Pain in extremityMusculoskeletal stiffness
Neck painGeneral disorders and administration site conditions Injection site reaction** Feeling hot
PyrexiaNo adverse reactions were reported after intrathecal administration of Iomeprol both in clinical trials and in the post-marketing surveillance.
4.8.3 Administration to body cavities
After injection of an iodinated contrast media in body cavities, contrast media are slowly absorbed from the area of administration into the systemic circulation and subsequently cleared by renal elimination.
Blood amylase increased is common following ERCP. Very rare cases of pancreatitis have been described.
The reactions reported in cases of arthrography and fistulography usually represent irritative manifestations superimposed on pre-existing conditions of tissue inflammation.
Hypersensitivity reactions are rare, generally mild and in the form of skin reactions. However, the possibility of severe anaphylactoid reactions cannot be excluded.
As with other iodinated contrast media, pelvic pain and malaise may occur after hysterosalpingography.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary/ intrathecal contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Following intrathecal administration to animals, iomeprol is completely cleared from the CSF and passes into the plasma compartment.
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of container
Colourless Type I or Type II glass bottles with rubber/aluminium cap.
Quantities of 20, 30, 50, 75, 100, 150, 200 or 250 ml of solution.6.6 Special precautions for disposal and other handling
Bottles containing contrast media solution are not intended for the withdrawal of multiple doses. The rubber stopper should never be pierced more than once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present.
The contrast medium should not be drawn into the syringe until immediately before use. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Sterile techniques must be used with any spinal puncture or intravascular injection, and with catheters and guidewires. If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
It is desirable that solutions of contrast media for intravascular and intrathecal use should be at body temperature when injected.
Any residue of contrast medium in the syringe must be discarded. Solutions not used in one examination session or waste material, such as the connecting tubes, should be disposed in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
11 December 1992 / 29 December 1998
10. DATE OF REVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 350, solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 71.44% w/v of iomeprol equivalent to 35% iodine or 350mg iodine/ml.
For the full list of excipients, see section 6.1.For excipients, see 6.1.
X-ray contrast medium used for:
- peripheral arteriography
- venography
- aortography
- angiocardiography and left ventriculography
- coronary arteriography
- visceral arteriography
- digital subtraction angiography
- computed tomography enhancement
- urography
- dacryocystography
- sialography
- fistulography
- galactography
4.2 Posology and method of administration
* Repeat as necessary
* * According to body size and ageperipheral arteriography adults 10 - 90ml * children * * venography adults 10 - 100ml* max 250ml
10 - 50ml upper extremity
50 - 100 lower extremityaortography adults 50 - 80ml children * * angiocardiography and left ventriculography adults 30 - 80ml max 250ml children * * coronary arteriography adults 4 - 10ml per artery * visceral arteriography adults 5 - 50ml* or according to type of examination; max 250ml children * *
digital subtraction angiographyintravenous adults 30 - 60ml* max 250ml
computed tomographybrain adults 50 - 150ml children * * body adults 40 - 150ml max 250ml children * *
Urographyintravenous adults 50 - 150ml neonates 3 - 4.8ml/kg babies 2.5 - 4ml children 1 - 2.5ml/kg or * arthrography adults up to 10ml dacryocystography adults 3 - 8ml sialography adults 1 - 3ml fistulography adults 1 - 50ml galactography adults 0.2 - 1.5ml In elderly patients the lowest effective dose should be used.
Unless otherwise instructed by the doctor, a normal diet may be maintained on the day of the examination.The X ray can be taken up to 60 minutes following injection.
Hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
Hydration
Patients must be well hydrated, and any relevant abnormalities of fluid or electrolyte balance should be corrected prior to and following contrast media injection. Especially patients with diabetes mellitus, polyuria, oligouria, hyperuricaemia, infants, small children, and elderly patients, should not be exposed to dehydration. Also patients with severely compromised hepatic and renal impairment are more at risk. Caution should be exercised in hydrating patients with underlying conditions that may be worsened by fluid overload, including congestive heart failure.Rehydration prior to use of iomeprol is recommended in patients with sickle cell disease.
Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighted against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in severe cardiac disease particularly heart failure and coronary artery disease. Reactions may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias.
In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased. In these cases the risks associated with the catheterization procedure are increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of contrast medium into cerebral tissue possibly leading to CNS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intra-arterial administration. Premedication with an alpha and beta receptor blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal impairment
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children: Infants up to 1 year, especially the new-born, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates 7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.
Elderly: There is special risk of reactions involving the circulatory system such that myocardial ischaemia, major arrhythmias and extrasystoles are more likely to occur. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.
Precautions for dedicated exams
Angiography
Non ionic contrast media have less antiocoagulant activity in vitro than ionic media. Meticulous attention should therefore be paid to angiographic technique. Non ionic media should not be allowed to remain in contact with blood in a syringe, and intravascular catheters should be flushed frequently to minimise the risk of clotting which, rarely, has led to serious thromboembolic complications.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
Venography
Special care is required when venography is performed in patients with thrombosis, phlebitis, severe ischaemic disease, local infection or a totally obstructed artero-venous system.4.5 Interaction with other medicaments and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenotiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.Breastfeeding
No human data exist concerning the excretion of iomeprol in breast milk. Animal studies have demonstrated that the excretion of iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.
Stopping breastfeeding is unnecessary.4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
4.8.1 Intravascular administration
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia, Haemolytic anaemia Immune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
Tachycardia
ExtrasystolesCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalCoronary artery thrombosis and coronary artery embolism have been reported as a complication of coronary catheterization procedures.
Vasospasm and consequent ischaemia have been observed during intra-arterial injections of contrast medium, in particular after coronary and cerebral angiography often procedurally related and possibly triggered by the tip of the catheter or excess catheter pressure.
As with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
4.8.2 Administration to body cavities
After injection of an iodinated contrast media in body cavities, contrast media are slowly absorbed from the area of administration into the systemic circulation and subsequently cleared by renal elimination.
Blood amylase increased is common following ERCP. Very rare cases of pancreatitis have been described.
The reactions reported in cases of arthrography and fistulography usually represent irritative manifestations superimposed on pre-existing conditions of tissue inflammation.
Hypersensitivity reactions are rare, generally mild and in the form of skin reactions. However, the possibility of severe anaphylactoid reactions cannot be excluded.
As with other iodinated contrast media, pelvic pain and malaise may occur after hysterosalpingography.Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. No other drug should be mixed with the contrast medium.
6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of containers
Colourless Type I or Type II glass bottles with rubber/aluminium cap.
Quantities of 20, 30, 50, 75, 100, 150, 200 or 250 ml of solution.6.6 Special precautions for disposal and other handling
Bottles containing contrast media solution are not intended for the withdrawal of multiple doses. The rubber stopper should never be pierced more than once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present.
The contrast medium should not be drawn into the syringe until immediately before use. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Sterile techniques must be used with any spinal puncture or intravascular injection, and with catheters and guidewires. If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
It is desirable that solutions of contrast media for intravascular and intrathecal use should be at body temperature when injected.
Any residue of contrast medium in the syringe must be discarded. Solutions not used in one examination session or waste material, such as the connecting tubes, should be disposed in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
11 December 1992 / 29 December 1998
10. DATE OFREVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 400, solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 81.65% w/v of iomeprol equivalent to 40% iodine or 400mg iodine/ml.
For the full list of excipients, see section 6.1.For excipients, see 6.1.
X-ray contrast medium used for:
- peripheral arteriography
- aortography
- angiocardiography and left ventriculography
- coronary arteriography
- visceral arteriography
- digital subtraction angiography
- computed tomography enhancement
- urography
- dacryocystography
- sialography
- fistulography
- galactography
4.2 Posology and method of administration
* Repeat as necessary
* * According to body size and ageperipheral arteriography adults 10 - 90ml * children * * aortography adults 50 - 80ml children * * angiocardiography and left ventriculography adults 30 - 80ml max 250ml children * * coronary arteriography adults 4 - 10ml per artery * visceral arteriography adults 5 - 50ml* or according to type of examination; children * *
digital subtraction angiographyintravenous adults 30 - 60ml* max 250ml
computed tomographybody adults 40 - 150ml max 250ml children * *
urographyintravenous adults 50 - 150ml neonates 3 - 4.8ml/kg babies 2.5 - 4ml children 1 - 2.5ml/kg or * dacryocystography adults 3 - 8ml sialography adults 1 - 3ml fistulography adults 1 - 50ml galactography adults 0.2 - 1.5ml In elderly patients the lowest effective dose should be used.
Unless otherwise instructed by the doctor, a normal diet may be maintained on the day of the examination.The X ray can be taken up to 60 minutes following injection.
Hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
Hydration
Patients must be well hydrated, and any relevant abnormalities of fluid or electrolyte balance should be corrected prior to and following contrast media injection. Especially patients with diabetes mellitus, polyuria, oligouria, hyperuricaemia, infants, small children, and elderly patients, should not be exposed to dehydration. Also patients with severely compromised hepatic and renal impairment are more at risk. Caution should be exercised in hydrating patients with underlying conditions that may be worsened by fluid overload, including congestive heart failure.Rehydration prior to use of iomeprol is recommended in patients with sickle cell disease.
Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatmen Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighted against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in severe cardiac disease particularly heart failure and coronary artery disease. Reactions may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias.
In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased. In these cases the risks associated with the catheterization procedure are increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of the contrast medium into cerebral tissue possibly leading to CMS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intra-arterial administration. Premedication with an alpha and beta receptor blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal impairment
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children: Infants up to 1 year, especially the newborn, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates 7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.
Elderly: There is special risk of reactions involving the circulatory system such that myocardial ischaemia, major arrhythmias and extrasystoles are more likely to occur. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.
Precautions for dedicated exams
Angiography
Non ionic contrast media have less antiocoagulant activity in vitro than ionic media. Meticulous attention should therefore be paid to angiographic technique. Non ionic media should not be allowed to remain in contact with blood in a syringe, and intravascular catheters should be flushed frequently to minimise the risk of clotting which, rarely, has led to serious thromboembolic complications.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
Venography
Special care is required when venography is performed in patients with thrombosis, phlebitis, severe ischaemic disease, local infection or a totally obstructed artero-venous system.4.5 Interaction with other medicaments and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenotiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.
Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible riskBreastfeeding
No human data exist concerning the excretion of iomeprol in breast milk. Animal studies have demonstrated that the excretion of iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.Stopping breastfeeding is unnecessary.
4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.4.8.1 Intravascular administration
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia, Haemolytic anaemia Immune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
Tachycardia
ExtrasystolesCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalCoronary artery thrombosis and coronary artery embolism have been reported as a complication of coronary catheterization procedures.
Vasospasm and consequent ischaemia have been observed during intra-arterial injections of contrast medium, in particular after coronary and cerebral angiography often procedurally related and possibly triggered by the tip of the catheter or excess catheter pressure.
As with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
4.8.2 Administration to body cavities
After injection of an iodinated contrast media in body cavities, contrast media are slowly absorbed from the area of administration into the systemic circulation and subsequently cleared by renal elimination.
Blood amylase increased is common following ERCP. Very rare cases of pancreatitis have been described.
The reactions reported in cases of arthrography and fistulography usually represent irritative manifestations superimposed on pre-existing conditions of tissue inflammation.
Hypersensitivity reactions are rare, generally mild and in the form of skin reactions. However, the possibility of severe anaphylactoid reactions cannot be excluded.
As with other iodinated contrast media, pelvic pain and malaise may occur after hysterosalpingography.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. No other drug should be mixed with the contrast medium.
6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of containers
Colourless Type I or Type II glass bottles with rubber/aluminium cap.
Quantities of 20, 30, 50, 75, 100, 150, 200 or 250 ml of solution.6.6 Special precautions for disposal and other handling
Bottles containing contrast media solution are not intended for the withdrawal of multiple doses. The rubber stopper should never be pierced more than once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present.
The contrast medium should not be drawn into the syringe until immediately before use. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Sterile techniques must be used with any spinal puncture or intravascular injection, and with catheters and guidewires. If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
It is desirable that solutions of contrast media for intravascular and intrathecal use should be at body temperature when injected.
Any residue of contrast medium in the syringe must be discarded. Solutions not used in one examination session or waste material, such as the connecting tubes, should be disposed in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
11 December 1992 / 29 December 1998
10. DATE OF REVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 300, solution for injection, multi-dose container
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 61.24% w/v of Iomeprol equivalent to 30% iodine or 300 mg iodine/ml.
For the full list of excipients, see section 6.1.
Solution for injection.
A clear colourless to pale yellow solution supplied in glass multi-dose container.X-ray contrast medium used for computed tomography enhancement, including CTA (CT Angiography).
4.2 Posology and method of administration
* According to body size and age brain adults 50 - 150ml children * body adults 40 - 150ml max 250ml children * In elderly patients the lowest effective dose should be used.
Hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
A normal diet should be maintained until the patient refrains from eating 2 hours before the procedure.
Hydration
Any severe disorders of water and electrolyte balance must be corrected prior to administration. Adequate hydration must be ensured particularly in patients with diabetes mellitus, polyuria, oliguria and hyperuricaemia; also in babies, small children and the elderly. Rehydration prior to use of Iomeprol is recommended in patients with sickle cell disease.Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighed against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in patients with severe cardiac disease particularly heart failure and coronary artery disease. Cardiac manifestations may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias. In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of the contrast medium into cerebral tissue possibly leading to CMS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intravascular administration. Premedication with an alpha and beta receptor-blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal failure
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters, in particular before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
- avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children:
Infants up to 1 year, especially the new-born, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an
iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates
7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.
Elderly:
The elderly are at special risk of reactions due to reduced physiological functions, especially when high dosage of contrast media is used. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
4.5 Interaction with other medicinal products and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to Iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenothiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of Iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.
Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible risk.Breastfeeding
No human data exist concerning the excretion of Iomeprol in breast milk. Animal studies have demonstrated that the excretion of Iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.Stopping breastfeeding is unnecessary.
4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia,
Haemolytic anaemiaImmune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
TachycardiaCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Extrasystoles
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalAs with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary/ intrathecal contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered Iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of Iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and Iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that Iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Five years
The maximum use time after a bottle stopper has been pierced is 10 hours.6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of container
Colourless type I or type II glass bottles with chlorobutyl or bromobutyl rubber stopper/aluminium cap containing 500 ml of solution.
Boxes of 1, 5 and 6 bottles.6.6 Special precautions for disposal and other handling
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present. The stopper should be pierced only once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Multi-dose containers should be used only in conjunction with an automatic injector which has been approved for multipatient use.
After each patient, the connector between the injector and the patient should be replaced. All other devices should be replaced following the injector manufacturer’s instructions. In any case, strictly follow the manufacturer’s instructions.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
10. DATE OF REVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 350, solution for injection, multi-dose container
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 71.44% w/v of Iomeprol equivalent to 35% iodine or 350 mg iodine/ml.
For the full list of excipients, see section 6.1.
Solution for injection.
A clear colourless to pale yellow solution supplied in glass multi-dose container.X-ray contrast medium used for computed tomography enhancement, including CTA (CT Angiography).
4.2 Posology and method of administration
* According to body size and age brain adults 50 - 150ml children * body adults 40 - 150ml max 250ml children * In elderly patients the lowest effective dose should be used.
Hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
A normal diet should be maintained until the patient refrains from eating 2 hours before the procedure.
Hydration
Any severe disorders of water and electrolyte balance must be corrected prior to administration. Adequate hydration must be ensured particularly in patients with diabetes mellitus, polyuria, oliguria and hyperuricaemia; also in babies, small children and the elderly. Rehydration prior to use of Iomeprol is recommended in patients with sickle cell disease.Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighed against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in patients with severe cardiac disease particularly heart failure and coronary artery disease. Cardiac manifestations may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias. In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of contrast medium into cerebral tissue possibly leading to CNS disorders. There is a possibility of a reduced seizure threshold in alcoholics.
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intravascular administration. Premedication with an alpha and beta receptor-blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal failure
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters, before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
- avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children: Infants up to 1 year, especially the new-born, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an
iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates
7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.Elderly:
The elderly are at special risk of reactions due to reduced physiological functions, especially when high dosage of contrast media is used. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.
Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
4.5 Interaction with other medicinal products and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to Iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenothiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.
It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of Iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.
Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible risk.Breastfeeding
No human data exist concerning the excretion of Iomeprol in breast milk. Animal studies have demonstrated that the excretion of Iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.
Stopping breastfeeding is unnecessary.4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia,
Haemolytic anaemiaImmune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
TachycardiaCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Extrasystoles
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalAs with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary/ intrathecal contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered Iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of Iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and Iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that Iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Five years
The maximum use time after a bottle stopper has been pierced is 10 hours.6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of containers
Colourless type I or type II glass bottles with chlorobutyl or bromobutyl rubber stopper/aluminium cap containing 500 ml of solution.
Boxes of 1, 5 and 6 bottles.6.6 Special precautions for disposal and other handling
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present. The stopper should be pierced only once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Multi-dose containers should be used only in conjunction with an automatic injector which has been approved for multipatient use.
After each patient, the connector between the injector and the patient should be replaced. All other devices should be replaced following the injector manufacturer’s instructions. In any case, strictly follow the manufacturer’s instructions.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
10. DATE OF REVISION OF THE TEXT
-
MEDICATION GUIDE
1. NAME OF THE MEDICINAL PRODUCT
Iomeron 400, solution for injection, multi-dose container
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Contains 81.65% w/v of Iomeprol equivalent to 40% iodine or 400 mg iodine/ml.
For the full list of excipients, see section 6.1.
Solution for injection.
A clear colourless to pale yellow solution supplied in glass multi-dose container.X-ray contrast medium used for computed tomography enhancement, including CTA (CT Angiography).
4.2 Posology and method of administration
* According to body size and age computed tomography body adults 40 - 150ml max 250ml children * In elderly patients the lowest effective dose should be used.
Hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings and special precautions for use
In consideration of possible complications, the patient should be kept under observation for at least 30 minutes after the examination.
Extreme caution during injection of contrast media is necessary to avoid extravasation.
A normal diet should be maintained until the patient refrains from eating 2 hours before the procedure.
Hydration
Any severe disorders of water and electrolyte balance must be corrected prior to administration. Adequate hydration must be ensured particularly in patients with diabetes mellitus, polyuria, oliguria and hyperuricaemia; also in babies, small children and the elderly. Rehydration prior to use of Iomeprol is recommended in patients with sickle cell disease.
Special population
Hypersensitivity to iodinated contrast media, allergic predisposition
A positive history of allergy, asthma or untoward reaction during previous similar investigations indicates a need for extra caution since, as with other contrast media, this product may provoke anaphylaxis or other manifestations of allergy with nausea, vomiting, dyspnoea, erythema, urticaria and hypotension. The benefits should clearly outweigh the risks in such patients and appropriate resuscitative measures should be immediately available. The primary treatments are as follows:Effect Major Symptoms Primary Treatment Vasomotor effect warmth
nausea/vomitingreassurance Cutaneous scattered hives
severe urticariaH1-antihistamines
H2-antihistaminesBronchospastic wheezing oxygen
Beta-2-agonist inhalersAnaphylactoid angioedema oxygen reaction urticaria
bronchospasm
hypotensioniv fluids
adrenergics (iv epinephrine)
Inhaled beta-2-adrenergicsantihistamines (H1-and H2- blockers)
corticosteroidsHypotensive hypotension iv fluids Vagal reaction hypotension
bradycardiaiv fluids
iv atropineFrom: Bush WH; The Contrast Media Manual; Katzburg RW Ed.; Williams and Wilkins; Baltimore 1992; Chapter 2 p 23
The risk of bronchospasm-inducing reactions in asthmatic patients is higher after contrast media administration, especially in patients taking beta-blockers.
Hypersensitivity testing
In patients with suspected or known hypersensitivity to contrast media, sensitivity test doses are not recommended, as severe or fatal reactions to contrast media are not predictable from sensitivity test.Myelomatosis or paraproteinaemias are conditions predisposing to renal impairment following CM administration. The benefits of the use of a contrast-enhanced procedure should be carefully weighed against the possible risk. Adequate hydration and monitoring of renal function are recommended after CM administration.
Cardiovascular diseases
Care should be taken in patients with severe cardiac disease particularly heart failure and coronary artery disease. Cardiac manifestations may include pulmonary oedema, haemodynamic changes, ischaemic ECG changes and arrhythmias. In severe, chronic hypertension the risk of renal damage following administration of a contrast medium is increased.The product should be used with caution in patients with hyperthyroidism or goitre. Use may interfere with thyroid function tests.
The administration of iodinated contrast media may aggravate myasthenia signs and symptoms.
CNS Disorders
Particular care is needed in patients with acute cerebral infarction, acute intracranial haemorrhage and any conditions involving damage to the blood brain barrier, brain oedema or acute demyelination. Convulsive seizures are more likely in patients with intracranial tumours or metastases or with a history of epilepsy.Neurological symptoms related to cerebrovascular diseases, intracranial tumours/metastases or degenerative or inflammatory pathologies may be exacerbated.
There is an increased risk of transient neurological complications in patients with symptomatic cerebrovascular disease eg stroke, transient ischaemic attacks. Cerebral ischaemic phenomena may be caused by intravascular injection.
Anticonvulsant therapy should not be discontinued.
In acute and chronic alcoholism the increase in blood brain barrier permeability facilitates the passage of the contrast medium into cerebral tissue possibly leading to CMS disorders. There is a possibility of a reduced seizure threshold in alcoholics
In patients with a drug addiction there is also the possibility of a reduced seizure threshold.
Patients with phaeochromocytoma may develop severe, occasionally uncontrollable hypertensive crises during intravascular administration. Premedication with an alpha and beta receptor-blocker is recommended in these patients. Pronounced excitement, anxiety and pain can cause side effects or intensify reaction to the contrast medium. A sedative may be given.
Renal failure
In patients with moderate to severe impairment of renal function, attention should be paid to renal function parameters, before re-examining the patient with a contrast media.
Preventive measures include:- identification of high-risk patients;
- ensuring adequate hydration before CM administration, preferably by maintaining i.v. infusion before and during the procedure and until the CM has been cleared by the kidneys;
- avoiding whenever possible, the administration of nephrotoxic drugs or major surgery or procedure such as renal angioplasty, until the CM has been cleared;
A combination of severe hepatic and renal impairment delays excretion of the contrast medium therefore such patients should not be examined unless absolutely necessary.
Diabetes mellitus
Care should be taken in renal impairment and diabetes. In these patients it is important to maintain hydration in order to minimise deterioration in renal function.
The presence of renal damage in diabetic patients is one of the factors predisposing to renal impairment following contrast media administration. This may precipitate lactic acidosis in patients who are taking metformin (see section 4.5 - Interaction with medicaments and other forms of interaction).Children: Infants up to 1 year, especially the newborn, are particularly susceptible to electrolyte imbalance and haemodynamic alterations. Care should be taken regarding the dosage used.
Transient hypothyroidism may occur in neonates when the mother or the neonate has received an
iodinated contrast agent. Thyroid function tests (usually TSH and T4) are recommended in neonates
7-10 days and 1 month after exposure to Iomeron especially in preterm neonates.Elderly:
The elderly are at special risk of reactions due to reduced physiological functions, especially when high dosage of contrast media is used. A combination of neurological disturbances and vascular pathologies present a serious complication. The probability of acute renal insufficiencies is higher in these people.Intravascular administration should be performed if possible with the patient lying down. The patient should be kept in this position and closely observed for at least 30 minutes after the procedure since the majority of severe incidents occur with this time.
4.5 Interaction with other medicinal products and other forms of interaction
Use of the product may interfere with tests for thyroid function. Vasopressor agents should not be administered prior to Iomeprol.
Treatment with drugs that lower the seizure threshold such as certain neuroleptics (MAO inhibitors, tricyclic antidepressants), analeptics, and anti-emetics and phenothiazine derivatives should be discontinued 48 hours before the examination. Treatment should not be resumed until 24 hours post-procedure.It has been reported that cardiac and/or hypertensive patients under treatment with diuretics, ACE-inhibitors, and/or beta blocking agents are at higher risk of adverse reactions when administered iodinated contrast media.
Beta-blockers may impair the response to treatment of bronchospasm induced by contrast medium.
Patients with normal renal function can continue to take metformin normally. In diabetic patients with diabetic nephropathy, under treatment with metformin and with moderate renal impairment, metformin should be stopped at the time of, or prior to the procedure and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal In emergency patients in whom renal function is either impaired or unknown, the physician shall weigh out risk and benefit of an examination with a contrast medium and take precautions. Metformin should be stopped from time of contrast medium administration. After the procedure the patient should be monitored for signs of lactic acidosis. Metformin should be restarted 48 hours after contrast medium if serum creatinine/eGFR is unchanged from the pre-imaging level.
Allergy-like reactions to contrast media are more frequent and may manifest as delayed reactions in patients treated with immuno-modulators, like Interleukin-2 (IL-2).
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Appropriate investigations and measures should be taken when exposing women of child-bearing potential to any X-ray examination, whether with or without contrast medium.Pregnancy
Animal studies have not indicated any harmful effects with respect to the course of pregnancy or on the health of the unborn or neonate. The safety of Iomeprol in human pregnancy however has not been established. Therefore avoid in pregnancy unless there is no safer alternative.
Since, wherever possible, exposure to radiation should be avoided during pregnancy, the benefits of any X ray examination, whether with or without contrast material, should for this reason alone be carefully weighed against the possible risk.Breastfeeding
No human data exist concerning the excretion of Iomeprol in breast milk. Animal studies have demonstrated that the excretion of Iomeprol in breast milk is similar to that of other contrast agents and that these compounds are only minimally absorbed by the gastrointestinal tract of the young. Adverse effects on the nursing infant are therefore unlikely to occur.Stopping breastfeeding is unnecessary.
4.7 Effects on ability to drive and use machines
There is no known effect on the ability to drive and operate machines.
The use of iodinated contrast media may cause untoward side effects. They are usually mild to moderate and transient in nature. However, severe and life-threatening reactions sometimes leading to death have been reported. In most cases, reactions occur within minutes of dosing but at times reactions may occur at later time.
Anaphylaxis (anaphylactoid/hypersensitivity reactions) may manifest with various symptoms, and rarely does any one patient develop all the symptoms. Typically, in 1 to 15 min (but rarely after as long as 2 h), the patient complains of feeling abnormal, agitation, flushing, feeling hot, sweating increased, dizziness, increased lacrimation, rhinitis, palpitations, paresthesia, pruritus, sore throat and throat tightness, dysphagia, cough, sneezing, urticaria, erythema, mild localised oedema, angioneurotic oedema and dyspnoea due to glottic/laryngeal/pharyngeal oedema and/or spasm manifesting with wheezing, and bronchospasm.
Nausea, vomiting, abdominal pain, and diarrhoea are also reported.
These reactions, which can occur independently of the dose administered or the route of administration, may represent the first signs of circulatory collapse.
Administration of the contrast medium must be discontinued immediately and, if needed, appropriate specific treatment urgently initiated via venous access.
Severe reactions involving the cardiovascular system, such as vasodilatation, with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness progressing to respiratory and/or cardiac arrest may result in death. These events can occur rapidly and require full and aggressive cardio-pulmonary resuscitation.
Primary circulatory collapse can occur as the only and/or initial presentation without respiratory symptoms or without other signs or symptoms outlined above.The adverse reactions reported in clinical trials among 4,903 adult patients and from post-marketing surveillance are represented in the tables below by frequency and classified by MedDRA system organ class.
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.Adult patients involved in clinical trials with intravascular administration of Iomeprol were 4,515.
* Since the reactions were not observed during clinical trials with 4515 patients, best estimate is that their relative occurrence is rare ( ≥1/10,000 to <1/1000).
The most appropriate MedDRA term is used to describe a certain reaction and its symptoms and related conditions.
** Injection site reactions comprise injection site pain and swelling. In the majority of cases they are due to extravasation of contrast medium. These reactions are usually transient and result in recovery without sequelae. Cases of extravasation with inflammation, skin necrosis and even development of compartment syndrome have been reported.Adults System Organ Class Adverse Reactions Clinical Trials Post-marketing Surveillance Common
(≥1/100 t o <1/10)Uncommon
(≥1/1000 to <1/100)Rare
(≥1/10,000 to <1/1000)Frequency unknown* Blood and lymphatic system disorders Thrombocytopenia,
Haemolytic anaemiaImmune system disorders Anaphylactoid reaction Psychiatric disorders Anxiety
Confusional stateNervous system disorders Headache Dizziness Presyncope Coma
Transient ischaemic attack
Paralysis
Syncope
Convulsion
Loss of consciousness
Dysarthria
Paraesthesia
Amnesia
Somnolence
Taste abnormalityEye disorders Blindness transient
Visual disturbance
Conjunctivitis
Lacrimation increased
PhotopsiaCardiac disorders Bradycardia
TachycardiaCardiac arrest
Myocardial infarction
Cardiac failure
Angina pectoris
Arrhythmia
Ventricular or atrial fibrillation
Atrioventricular block
Extrasystoles
Palpitations
CyanosisVascular disorders Hypertension Hypotension Circulatory collapse or shock
Hot flush
Flushing
PallorRespiratory, thoracic and mediastinal disorders Dyspnoea Respiratory arrest
Acute respiratory distress syndrome (ARDS)
Pulmonary oedema
Laryngeal oedema
Pharyngeal oedema
Bronchospasm
Asthma
Cough
Hyperventilation
Pharynx discomfort
Laryngeal discomfort
Rhinitis
DysphoniaGastrointestinal disorders Nausea
VomitingDiarrhoea
Abdominal pain
Salivary hypersecretion
Dysphagia
Salivary gland enlargementSkin and subcutaneous tissue disorders Erythema
Urticaria
PruritusRash Acute generalized exanthematous pustulosis
Angioedema
Cold sweat
Sweating increasedMusculoskeletal and connective tissue disorder Back pain Arthralgia Renal and urinary disorders Renal failure General disorders and administration site conditions Feeling hot Chest pain
Injection site warmth and painAsthenia
Rigors
PyrexiaInjection site reaction**
Coldness local
Fatigue
Malaise
ThirstInvestigations Blood creatinine increased Electrocardiogram ST segment elevation
Electrocardiogram abnormalAs with other iodinated contrast media, very rare cases of mucocutaneous syndromes, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome) and erythema multiforme, have been reported following the administration of Iomeprol injection.
There is limited experience with paediatric patients. The clinical trial paediatric safety database comprises 167 patients.
The Iomeprol safety profile is similar in children and adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.The effects of overdose on the pulmonary and cardiovascular systems may become life-threatening. Treatment consists of support of the vital functions and prompt use of symptomatic therapy. Iomeprol does not bind to plasma or serum proteins and is therefore dialyzable.
5.1 Pharmacodynamic properties
Iomeprol is a low osmolality, non-ionic organic molecule with radio-opacity conferred by an iodine content of 49% of the molecular weight. It is formulated for use as an intravascular/intracavitary/ intrathecal contrast medium in concentrations of up to 400mg iodine per ml. Even at this concentration the low viscosity allows delivery of high doses through thin catheters.
5.2 Pharmacokinetic properties
The pharmacokinetics of intravascularly administered Iomeprol are similar to those of other iodinated contrast media and conform to a two-compartment model with a rapid distribution and a slower elimination phase. In healthy subjects, the mean distribution and elimination half-lives of Iomeprol were 0.5 hours and 1.9 hours respectively.
Distribution volume is similar to that of extra cellular fluid. There is no significant serum protein binding and Iomeprol is not metabolized.
Elimination is almost exclusively through the kidneys (90% of the dose recovered in the urine within 96 hours of its administration) and is rapid (50% of an intravascularly administered dose within 2 hours).
Pre-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction.
Results from studies in rats, mice and dogs demonstrate that Iomeprol has an acute intravenous or intra-arterial toxicity similar to that of the other non ionic contrast media, as well as a good systemic tolerability after repeated intravenous administrations in rats and dogs.
trometamol
hydrochloric acid
water for injectionIn the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Five years
The maximum use time after a bottle stopper has been pierced is 10 hours.6.4 Special precautions for storage
Store below 30°C
Protect from light6.5 Nature and contents of containers
Colourless type I or type II glass bottles with chlorobutyl or bromobutyl rubber stopper/aluminium cap containing 500 ml of solution.
Boxes of 1, 5 and 6 bottles.6.6 Special precautions for disposal and other handling
Before use, examine the product to assure that the container and closure have not been damaged. Do not use the solution if it is discolored or particulate matter is present. The stopper should be pierced only once. The use of proper withdrawal cannulas for piercing the stopper and drawing up the contrast medium is recommended.
Multi-dose containers should be used only in conjunction with an automatic injector which has been approved for multipatient use.
After each patient, the connector between the injector and the patient should be replaced. All other devices should be replaced following the injector manufacturer’s instructions. In any case, strictly follow the manufacturer’s instructions.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Bracco UK Ltd
Magdalen Centre
The Oxford Science Park
Oxford, OX4 4GA
United Kingdom8. MARKETING AUTHORISATION NUMBER
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
10. DATE OF REVISION OF THE TEXT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-725 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 510 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-725-10 56 in 1 BOX 07/01/2022 02/01/2027 1 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-730 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 612 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-730-10 56 in 1 BOX 07/01/2022 02/01/2027 1 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:76381-730-20 10 in 1 BOX 07/01/2022 12/01/2026 2 200 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-735 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 714 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-735-10 56 in 1 BOX 07/01/2022 09/01/2026 1 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:76381-735-15 30 in 1 BOX 07/01/2022 02/01/2027 2 150 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 3 NDC:76381-735-20 10 in 1 BOX 07/01/2022 03/01/2027 3 200 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-740 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 816 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-740-10 10 in 1 BOX 07/01/2022 03/01/2025 1 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:76381-740-13 56 in 1 BOX 07/01/2022 09/01/2026 2 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 3 NDC:76381-740-16 100 in 1 BOX 07/01/2022 01/01/2027 3 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 4 NDC:76381-740-20 30 in 1 BOX 07/01/2022 02/01/2027 4 200 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-930 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 612 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-930-06 6 in 1 BOX 07/01/2022 02/01/2027 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-935 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 714 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-935-06 6 in 1 BOX 07/01/2022 02/01/2027 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:76381-935-09 9 in 1 BOX 07/01/2022 03/01/2027 2 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 IOMERON
iomeprol injection injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76381-940 Route of Administration INTRAVASCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOMEPROL (UNII: 17E17JBP8L) (IOMEPROL - UNII:17E17JBP8L) IOMEPROL 816 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76381-940-01 9 in 1 BOX 07/01/2022 03/01/2027 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:76381-940-03 9 in 1 BOX 07/01/2022 03/01/2025 2 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 3 NDC:76381-940-06 6 in 1 BOX 07/01/2022 02/01/2027 3 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/01/2022 Labeler - BIPSO GmbH (342104149) Registrant - BIPSO GmbH (342104149) Establishment Name Address ID/FEI Business Operations BioChem Labor für biologishe und chemische Analytik GmbH 318354230 ANALYSIS(76381-725, 76381-730, 76381-735, 76381-740, 76381-930, 76381-935, 76381-940) Establishment Name Address ID/FEI Business Operations SPIN S.p.A. 434967237 API MANUFACTURE(76381-725, 76381-730, 76381-735, 76381-740, 76381-930, 76381-935, 76381-940) Establishment Name Address ID/FEI Business Operations BIPSO GmbH 342104149 MANUFACTURE(76381-725, 76381-730, 76381-735, 76381-740, 76381-725, 76381-740, 76381-940) , ANALYSIS(76381-930, 76381-935, 76381-940, 76381-730, 76381-735, 76381-930, 76381-935)