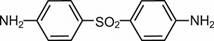Label: DAPSONE gel
- NDC Code(s): 72578-094-01, 72578-094-02, 72578-094-03
- Packager: Viona Pharmaceuticals Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DAPSONE GEL safely and effectively. See full prescribing information for DAPSONE GEL. DAPSONE gel, for topical use - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDapsone gel, 7.5%, is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Not for oral, ophthalmic, or intravaginal use. After the skin is gently washed and patted dry, apply approximately a pea-sized amount of dapsone gel, 7.5%, in a thin layer ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 7.5%. Each gram of dapsone gel, 7.5% contains 75 mg of dapsone in an white, off white to yellow gel with suspended particles.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hematological Effects - Methemoglobinemia - Cases of methemoglobinemia, with resultant hospitalization, have been reported postmarketing in association with twice daily dapsone gel, 5% ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo formal drug-drug interaction studies were conducted with dapsone gel, 7.5%. 7.1 Trimethoprim-Sulfamethoxazole - A drug-drug interaction study evaluated the effect of the use of dapsone gel ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on dapsone gel, 7.5%, use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. The systemic absorption ...
-
11 DESCRIPTIONDapsone gel, 7.5%, contains dapsone, a sulfone, in an aqueous gel base for topical dermatologic use. Dapsone gel, 7.5% is an white, off white to yellow gel with suspended particles. Chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of dapsone gel in treating acne vulgaris is not known. 12.3 Pharmacokinetics - In a pharmacokinetic study, male and female subjects 16 years ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Dapsone was not carcinogenic to rats when orally administered to females for 92 weeks or males for 100 weeks at dose levels up to 15 ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily use of dapsone gel, 7.5%, was assessed in two 12- week multicenter, randomized, double-blind, vehicle-controlled trials. Efficacy was assessed in a total of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDapsone gel, 7.5% is a white, off white to yellow gel with suspended particles. It is supplied in an airless pump containing a polypropylene bottle with a high density polyethylene ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Hematological Effects - Inform patients that methemoglobinemia can occur with topical dapsone treatment ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Lifesciences Ltd. Ahmedabad, India - Distributed by: Viona Pharmaceuticals Inc. Cranford, NJ 07016 - Rev.: 06/23
-
PATIENT PACKAGE INSERTPatient Information - Dapsone (dap' sone) Gel, 7.5% Important: For use on skin only (topical use). Do not use dapsone gel, 7.5% in your mouth, eyes, or vagina. What is dapsone ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELDapsone Gel, 7.5% 60 g - NDC 72578-094-02 - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information