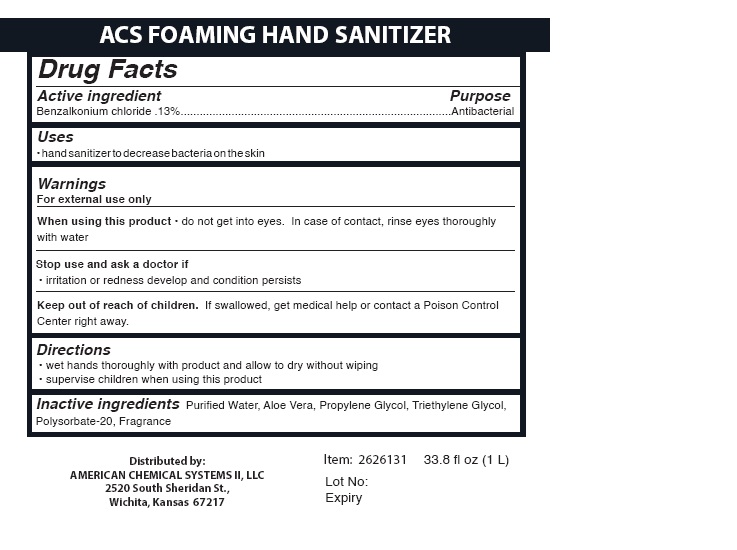
Label: FOAMING HAND SANITIZER- hand sanitizer liquid
- NDC Code(s): 75044-280-16, 75044-280-31
- Packager: American Chemical Systems II, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- ACS™ FOAMING HAND SANITIZER Labeling
-
INGREDIENTS AND APPEARANCE
FOAMING HAND SANITIZER
hand sanitizer liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75044-280 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 g in 1000 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA FLOWER (UNII: 575DY8C1ER) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRIETHYLENE GLYCOL (UNII: 3P5SU53360) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75044-280-31 3 in 1 CASE 09/01/2024 1 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:75044-280-16 2 in 1 CASE 09/01/2024 2 3800 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 09/01/2024 Labeler - American Chemical Systems II, LLC (016631058)