Label: METFORMIN HYDROCHLORIDE tablet
- NDC Code(s): 70518-2833-0, 70518-2833-1, 70518-2833-2, 70518-2833-3, view more
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 70010-063
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for METFORMIN HYDROCHLORIDE TABLETS ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: LACTIC ACIDOSIS
Close
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration(2.3),(2.7), Contraindications (4), Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin hydrochloride tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [ seeWarnings and Precautions (5.1)]. -
1 INDICATIONS & USAGEMetformin hydrochloride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes ...
-
2 DOSAGE & ADMINISTRATION2.1 Adult Dosage - Metformin Hydrochloride Tablets - The recommended starting dose of metformin hydrochloride tablets are 500 mg orally twice a day or 850 mg once a day, given with ...
-
3 DOSAGE FORMS & STRENGTHSMetformin Hydrochloride Tablets, USP are available as: • Tablets: 500 mg - White to off-white, round, biconvex, film coated tablets debossed with - G 10 on one side and plain on the ...
-
4 CONTRAINDICATIONSMetformin hydrochloride tablets are contraindicated in patients with: Severe renal impairment (eGFR below 30 mL/min/1.73 m - 2) [ see Warnings and Precautions (5.1)] ...
-
5 WARNINGS AND PRECAUTIONS5.1 Lactic Acidosis - There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific ...
-
6 ADVERSE REACTIONSThe following adverse reactions are also discussed elsewhere in the labeling: Lactic Acidosis [ see Boxed WarningandWarnings and Precautions (5.1)]. Vitamin B12 - Deficiency ...
-
7 DRUG INTERACTIONSTable 3 presents clinically significant drug interactions with metformin hydrochloride tablets. Table 3: Clinically Significant Drug Interactions with Metformin Hydrochloride ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited data with metformin hydrochloride tablets in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or ...
-
10 OVERDOSAGEOverdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with ...
-
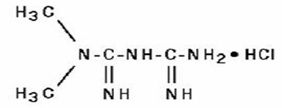
11 DESCRIPTIONMetformin hydrochloride tablets, USP contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin hydrochloride is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks ...
-
14 CLINICAL STUDIES14.1 Metformin Hydrochloride Tablets - Adult Clinical Studies - A double-blind, placebo-controlled, multicenter US clinical trial involving obese patients with type 2 diabetes mellitus whose ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetformin Hydrochloride Tablets, USP Available In: 500 mg White to off-white, round, biconvex, film coated tablets debossing G on 10 one side and plain on the other side. NDC: 70518-2833-00 - NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Lactic Acidosis: Explain the risks of lactic acidosis, its symptoms, and conditions that predispose ...
-
PATIENT PACKAGE INSERT. PATIENT INFORMATION - Metformin Hydrochloride Tablets - (met-FOR-min HYE-droe-KLOR-ide) Read the Patient Information that comes with metformin hydrochloride tablets before you start taking ...
-
.DRUG: Metformin Hydrochloride - GENERIC: Metformin Hydrochloride - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-2833-0 - NDC: 70518-2833-1 - NDC: 70518-2833-2 - NDC: 70518-2833-3 - NDC: 70518-2833-4 - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information