Label: MEMANTINE tablet
- NDC Code(s): 60687-173-11, 60687-173-57, 60687-184-11, 60687-184-57
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 55111-596, 55111-597
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEMANTINE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for MEMANTINE HYDROCHLORIDE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMemantine hydrochloride tablets are indicated for the treatment of moderate to severe dementia of the Alzheimer’s type.
-
2 DOSAGE AND ADMINISTRATIONThe recommended starting dose of memantine hydrochloride is 5 mg once daily. The dose should be increased in 5 mg increments to 10 mg/day (5 mg twice daily), 15 mg/day (5 mg and 10 mg as separate ...
-
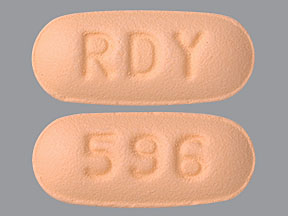
3 DOSAGE FORMS AND STRENGTHSMemantine hydrochloride tablets USP, 5 mg are orange colored, capsule shaped, biconvex, film coated tablets debossed with ‘RDY’ on one side and ‘596’ on other side. Memantine hydrochloride tablets ...
-
4 CONTRAINDICATIONSMemantine hydrochloride tablets are contraindicated in patients with known hypersensitivity to memantine hydrochloride or to any excipients used in the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Genitourinary Conditions - Conditions that raise urine pH may decrease the urinary elimination of memantine resulting in increased plasma levels of memantine [ see Drug Interactions ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Memantine hydrochloride was evaluated in eight double-blind placebo-controlled trials involving a total of 1862 dementia (Alzheimer’s disease, vascular dementia ...
-
7 DRUG INTERACTIONS7.1 Drugs that Make the Urine Alkaline - The clearance of memantine was reduced by about 80% under alkaline urine conditions at pH 8. Therefore, alterations of urine pH towards the alkaline ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of memantine hydrochloride in pregnant women. Adverse developmental effects ...
-
10 OVERDOSAGESigns and symptoms most often accompanying memantine overdosage in clinical trials and from worldwide marketing experience, alone or in combination with other drugs and/or alcohol, include ...
-
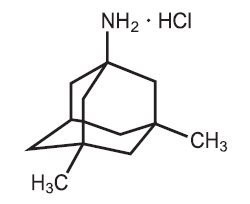
11 DESCRIPTIONMemantine hydrochloride is an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Persistent activation of central nervous system N-methyl-D-aspartate (NMDA) receptors by the excitatory amino acid glutamate has been hypothesized to contribute to the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of carcinogenicity in a 113-week oral study in mice at doses up to 40 mg/kg/day (10 times the maximum recommended ...
-
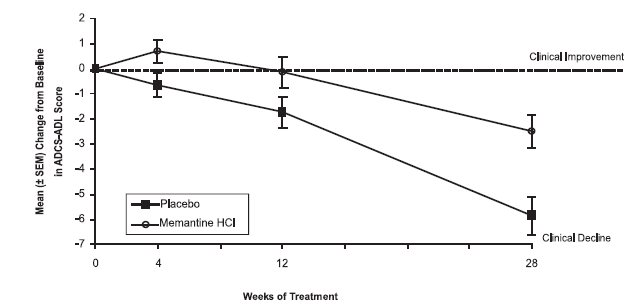
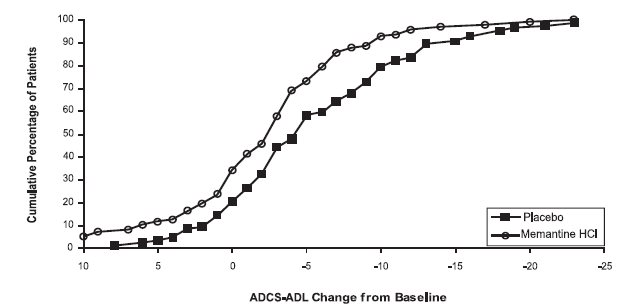
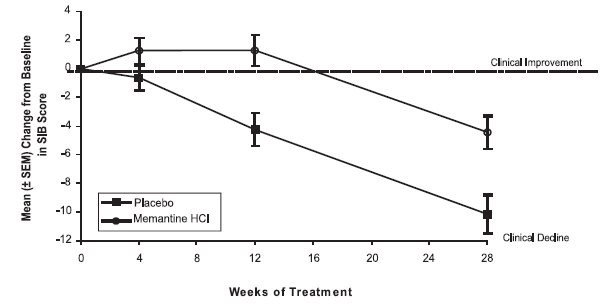
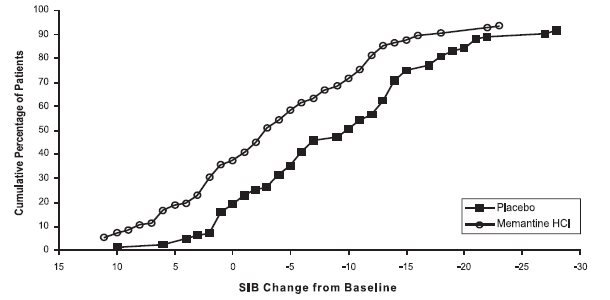
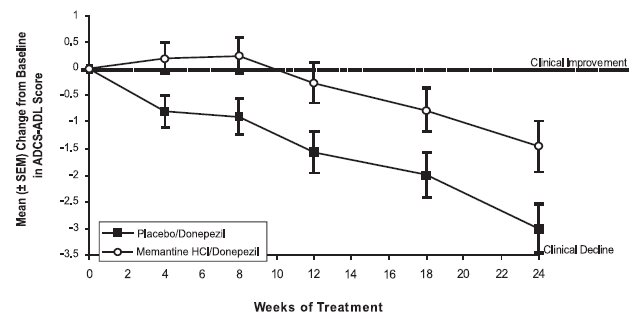
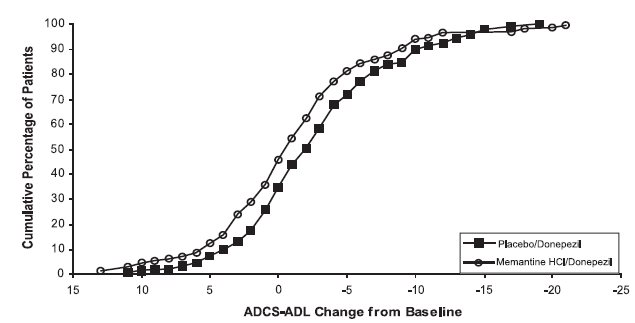
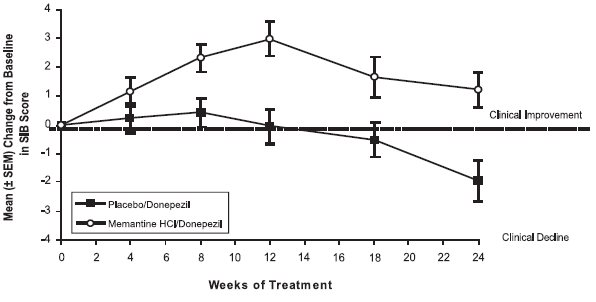
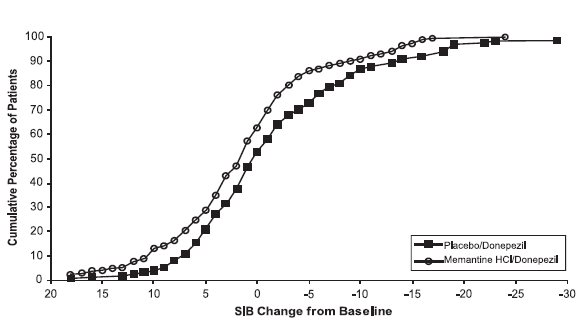
14 CLINICAL STUDIESThe effectiveness of memantine hydrochloride as a treatment for patients with moderate to severe Alzheimer’s disease was demonstrated in 2 randomized, double-blind, placebo-controlled clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMemantine hydrochloride tablets USP, 5 mg are orange colored, capsule shaped, biconvex, film coated tablets debossed with ‘RDY’ on one side and ‘596’ on other side. They are supplied in unit dose ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling ( Patient Information). To assure safe and effective use of memantine hydrochloride, the following information provided in the patient information ...
-
Patient InformationMemantine Hydrochloride Tablets, USP - (mem’ an teen hye” droe klor’ ide) Read this Patient Information that comes with memantine hydrochloride tablets before you start taking it and each time ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose blisters (see - How Supplied section) contain drug product from Dr. Reddy’s Laboratories Limited as follows: (5 mg / 60 UD) NDC 60687-173-57 packaged from ...
-
Package/Label Display Panel – Carton – 5 mgNDC 60687- 173-57 - Memantine - Hydrochloride - Tablets, USP - 5 mg - 60 Tablets (6 x 10) Rx Only - PHARMACIST: Dispense with Patient Information to each patient. Each ...
-
Package/Label Display Panel – Blister – 5 mgMemantine Hydrochloride - Tablet, USP - 5 mg
-
Package/Label Display Panel – Carton – 10 mgNDC 60687- 184-57 - Memantine - Hydrochloride - Tablets, USP - 10 mg - 60 Tablets (6 x 10) Rx Only - PHARMACIST: Dispense with the accompanying Patient - Information Sheet to ...
-
Package/Label Display Panel – Blister – 10 mgMemantine Hydrochloride - Tablet, USP - 10 mg
-
INGREDIENTS AND APPEARANCEProduct Information