Label: AMPICILLIN- ampicillin injection powder, for solution
- NDC Code(s): 23155-914-31, 23155-914-41, 23155-915-31, 23155-915-41, view more
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Rx onlyTo reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin and other antibacterial drugs, ampicillin should be used only to treat or prevent infections that ...
-
DESCRIPTION
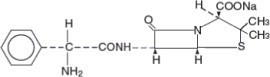
Ampicillin for Injection, USP the monosodium salt of [2S-[2α, 5α, 6β(S*)]]-6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid, is a synthetic ...
-
CLINICAL PHARMACOLOGY
Ampicillin for Injection, USP diffuses readily into most body tissues and fluids. However, penetration into the cerebrospinal fluid and brain occurs only when the meninges are inflamed ...
-
INDICATIONS AND USAGE
Ampicillin for Injection, USP is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the following conditions: Respiratory Tract Infections ...
-
CONTRAINDICATIONS
A history of a previous hypersensitivity reaction to any of the penicillins is a contraindication.
-
WARNINGS
Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions have been reported in patients on penicillin therapy. Although anaphylaxis is more frequent following parenteral therapy ...
-
PRECAUTIONS
General - The possibility of superinfections with mycotic organisms or bacterial pathogens should be kept in mind during therapy. In such cases, discontinue the drug and substitute appropriate ...
-
ADVERSE REACTIONS
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously ...
-
OVERDOSAGE
In cases of overdose, discontinue medication, treat symptomatically, and institute supportive measures as required. In patients with renal function impairment, ampicillin-class antibiotics can be ...
-
DOSAGE AND ADMINISTRATION
Infections of the respiratory tract and soft tissues. Patients weighing 40 kg (88 lbs) or more: 250 mg to 500 mg every 6 hours. Patients weighing less than 40 kg (88 lbs): 25 to 50 mg/kg/day in ...
-
HOW SUPPLIED
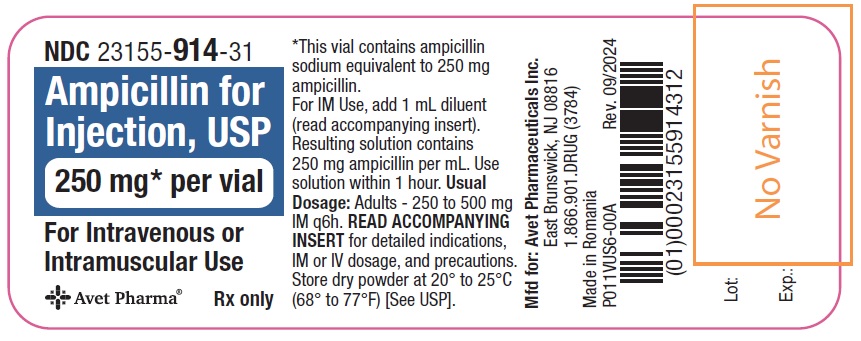
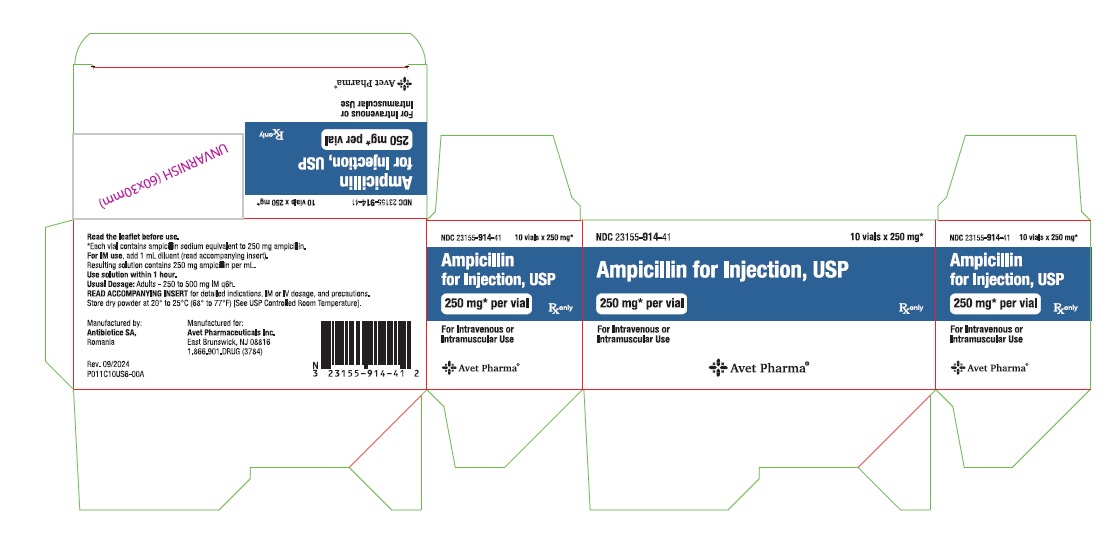
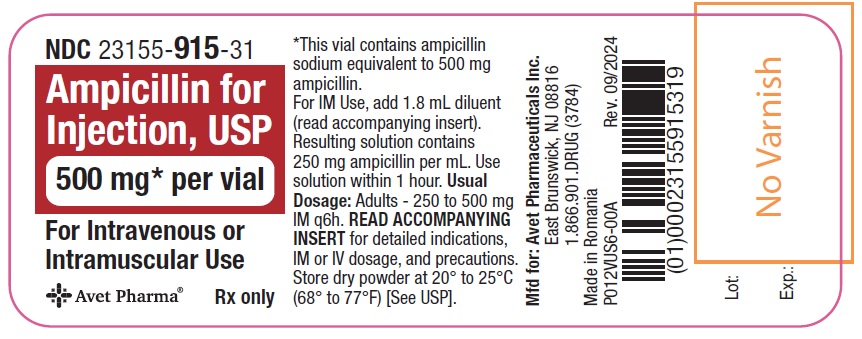
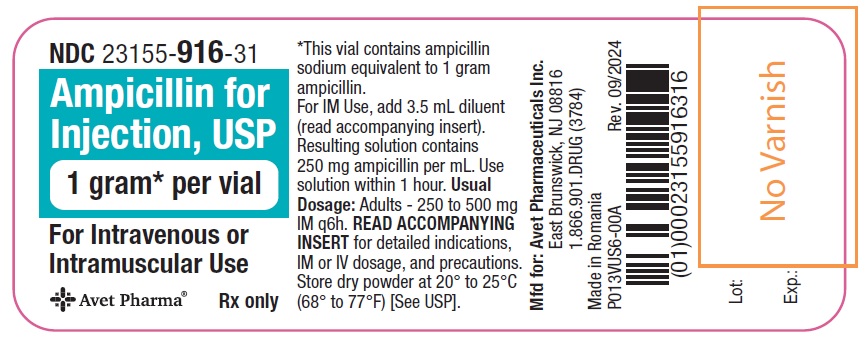
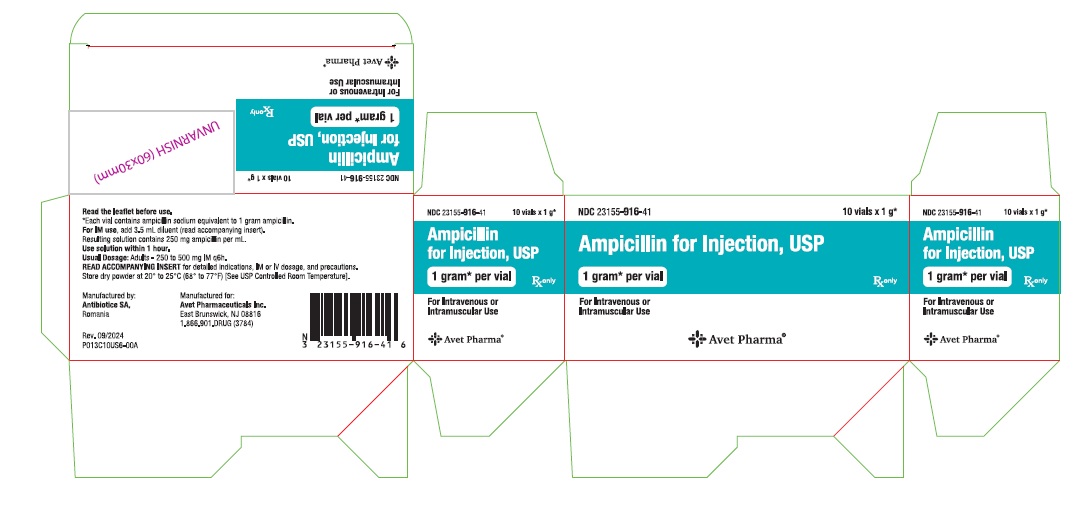
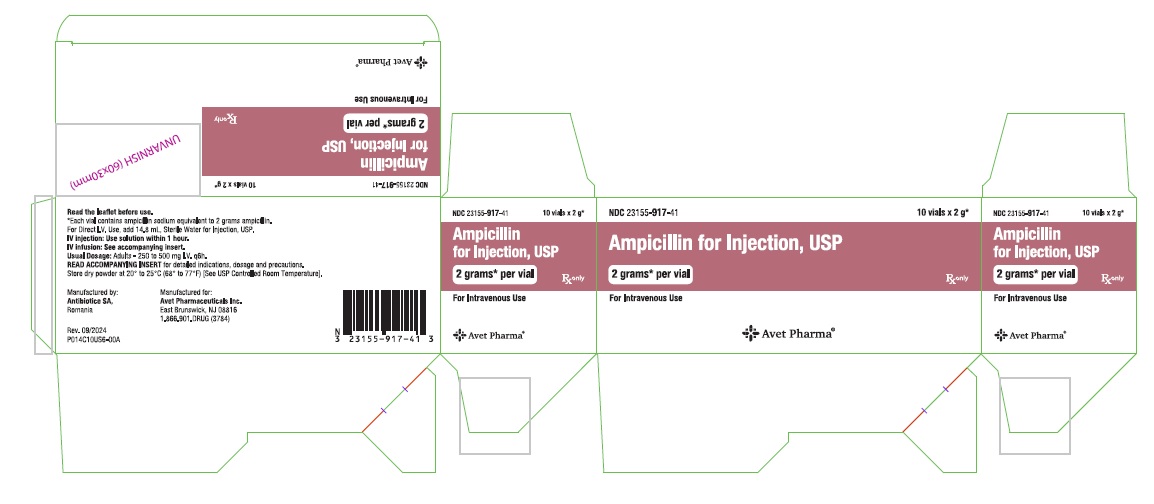
Ampicillin for Injection, USP, is supplied as follows: NDC Ampicillin for Injection, USP Package Factor - 23155-914-41 250 mg per vial 10 vials per carton - 23155-915-41 500 mg per vial 10 ...
-
Principal Display PanelNDC 23155-914-31 - Ampicillin for - Injection, USP - 250 mg* per vial - For Intravenous or Intramuscular Use - Rx only - Label - Carton - NDC 23155-915-31 - Ampicillin for - Injection ...
-
INGREDIENTS AND APPEARANCEProduct Information