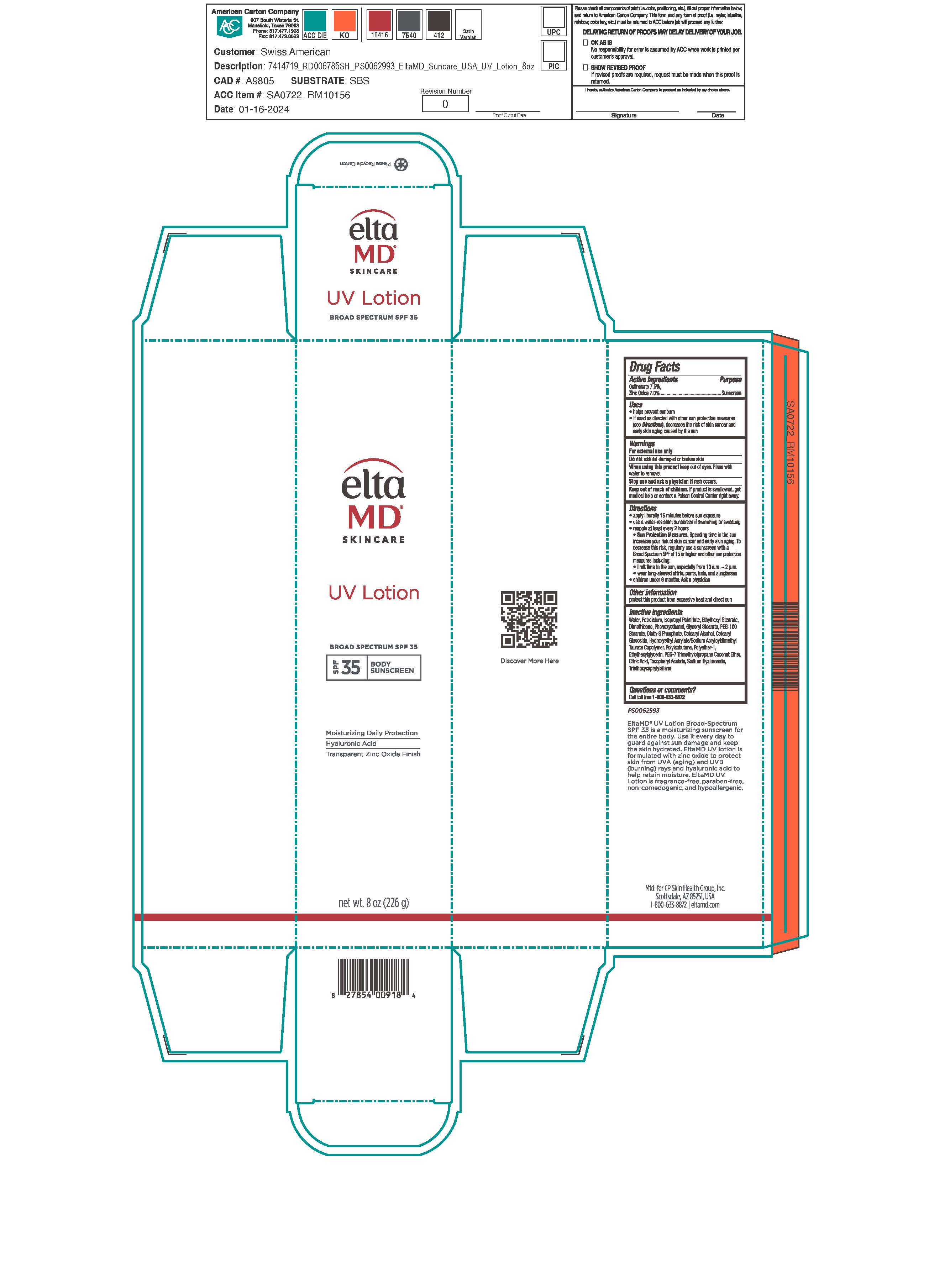
Label: ELTAMD UV SPF35- octinoxate, zinc oxide sunscreen lotion
- NDC Code(s): 72043-6808-8
- Packager: CP Skin Health Group, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active ingredients
- Indications & Uses:
- Indications & Uses:
- Keep out of reach of children
-
Directions
Apply liberally 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every 2 hours. Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am to 2 pm. Wear long-sleeve shirts, pants, hats, and sunglasses. Children under 6 months: ask a physician.
- Other information:
-
Inactive ingredients
water, petrolatum, isopropyl palmitate, ethylhexyl stearate, dimethicone, phenoxyethanol, glyceryl stearate, PEG-100 stearate, oleth-3 phosphate, cetearyl alcohol, cetearyl glucoside, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, polyisobutene, polyether-1, ethylhexylglycerin, PEG-7 trimethylolpropane coconut ether, citric acid, tocopheryl acetate, sodium hyaluronate, triethoxycaprylylsilane
- Questions?
- Labeling
-
INGREDIENTS AND APPEARANCE
ELTAMD UV SPF35
octinoxate, zinc oxide sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-6808 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 70 g in 1000 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PETROLATUM (UNII: 4T6H12BN9U) DIMETHICONE (UNII: 92RU3N3Y1O) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) OCTYL STEARATE (UNII: 772Y4UFC8B) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) GLYCERYL STEARATE/PEG-100 STEARATE (UNII: RD25J5V947) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONIC ACID (UNII: S270N0TRQY) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) PEG-7 TRIMETHYLOLPROPANE COCONUT ETHER (UNII: MVJ3AD73GG) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-6808-8 226 g in 1 TUBE; Type 0: Not a Combination Product 05/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2024 Labeler - CP Skin Health Group, Inc (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-6808)