Label: NOREPINEPHRINE BITARTRATE injection, solution, concentrate
- NDC Code(s): 70771-1724-1, 70771-1724-6
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 26, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
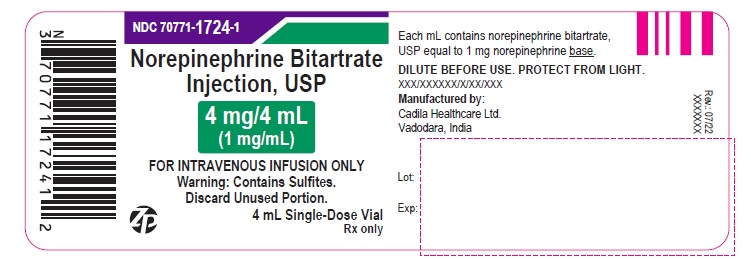
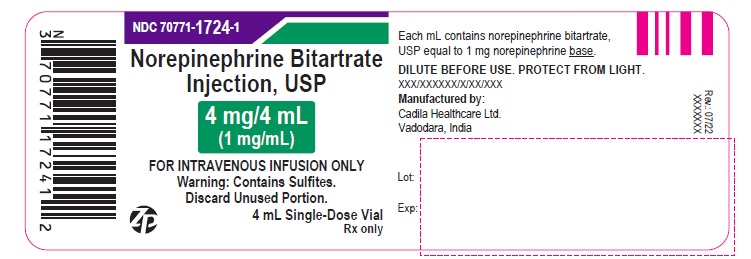
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Norepinephrine Bitartrate Injection, USP
4 mg/4 mL (1 mg/mL)
FOR INTRAVENOUS INFUSION ONLY.
STERILE INJECTION
Warning: This is potent drug. Dosage should be controlled by frequent determination of blood pressure.
Do not leave patient unattended during administration. Avoid extravasation. Read package insert carefully.
DILUTE BEFORE USE. DISCARD UNUSED PORTION. PROTECT FROM LIGHT.
Warning: Contains Sulfites.
10 X 4 mL Single-Dose Vials
Rx only

Norepinephrine Bitartrate Injection, USP
4 mg/4 mL (1 mg/mL)
FOR INTRAVENOUS INFUSION ONLY
Warning: Contains Sulfites.
DISCARD UNUSED PORTION.
4 mL Single-Dose Vial
Rx only
-
INGREDIENTS AND APPEARANCE
NOREPINEPHRINE BITARTRATE
norepinephrine bitartrate injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1724 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOREPINEPHRINE BITARTRATE (UNII: IFY5PE3ZRW) (NOREPINEPHRINE - UNII:X4W3ENH1CV) NOREPINEPHRINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM METABISULFITE (UNII: 4VON5FNS3C) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1724-6 10 in 1 CARTON 04/24/2024 1 NDC:70771-1724-1 4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216341 04/24/2024 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 873671928 MANUFACTURE(70771-1724) , ANALYSIS(70771-1724)