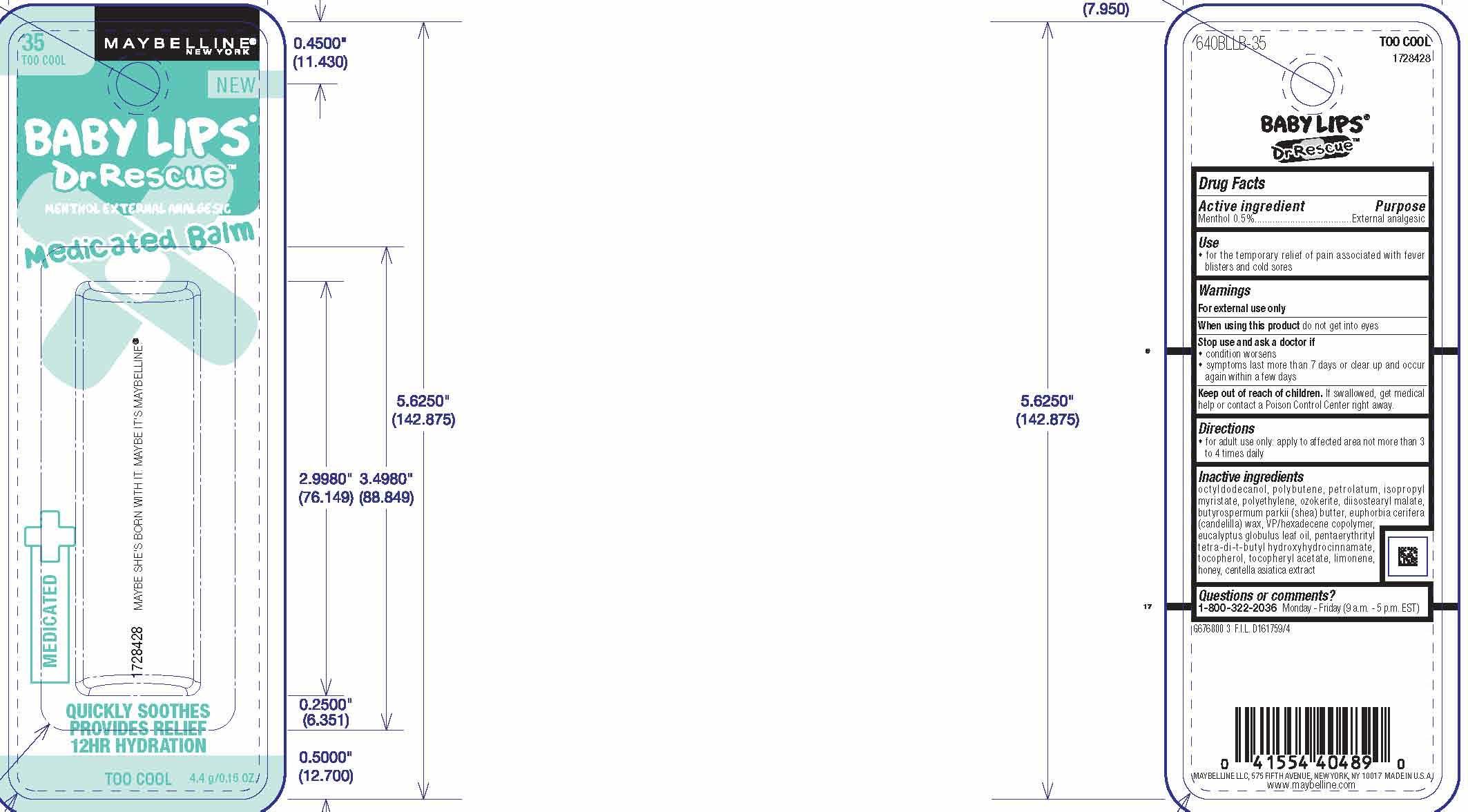
Label: MAYBELLINE NEW YORK BABY LIPS DR RESCUE MEDICATED BALM- menthol lipstick
- NDC Code(s): 49967-489-01
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
-
Inactive ingredients
octyldodecanol, polybutene, petrolatum, isopropyl myristate, polyethylene, ozokerite, diisostearyl malate, butyrospermum parkii (shea) butter, euphorbia cerifera (candelilla) wax, VP/hexadecene copolymer, eucalyptus globulus leaf oil, penaerythrityl tetra-di-t-butyl hydroxyhydrocinamate, tocopherol, tocopheryl acetate, limonene, honey, centella asiatica extract
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAYBELLINE NEW YORK BABY LIPS DR RESCUE MEDICATED BALM
menthol lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-489 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) SHEA BUTTER (UNII: K49155WL9Y) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-489-01 1 in 1 BLISTER PACK 10/01/2013 1 4.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/01/2013 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 624244349 manufacture(49967-489)