Label: MEGESTROL ACETATE suspension
- NDC Code(s): 24979-041-13
- Packager: Upsher-Smith Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEGESTROL ACETATE ORAL SUSPENSION safely and effectively. See full prescribing information for MEGESTROL ACETATE ORAL SUSPENSION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMegestrol acetate oral suspension is indicated for the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome ...
-
2 DOSAGE AND ADMINISTRATION2.1 Testing - Prior to Megestrol Acetate Oral Suspension Administration - Obtain a negative pregnancy test in females of reproductive potential prior to initiating treatment with megestrol ...
-
3 DOSAGE FORMS AND STRENGTHSMegestrol acetate oral suspension is milky white, lemon flavored, and contains 125 mg per mL.
-
4 CONTRAINDICATIONSHistory of hypersensitivity to megestrol acetate or any component of the formulation. Pregnancy [see Warnings and Precautions (5.2), Use in Specific Populations (8.1, 8.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 General - Effects on HIV viral replication have not been determined. Use with caution in patients with a history of thromboembolic disease. 5.2 Fetal Toxicity - Based on animal studies ...
-
6 ADVERSE REACTIONS6.1 Serious and Otherwise Important Adverse Reactions - The following serious reactions and otherwise important adverse drug reactions are discussed in greater detail in other sections of the ...
-
7 DRUG INTERACTIONS7.1 Indinavir - Due to the significant decrease in the exposure of indinavir by megestrol acetate, administration of a higher dose of indinavir should be considered when coadministering with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal data, megestrol acetate may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy [see Contraindications ...
-
10 OVERDOSAGENo serious unexpected side effects have resulted from studies involving megestrol acetate oral suspension administered in dosages as high as 1200 mg/day. In post-marketing experience, limited ...
-
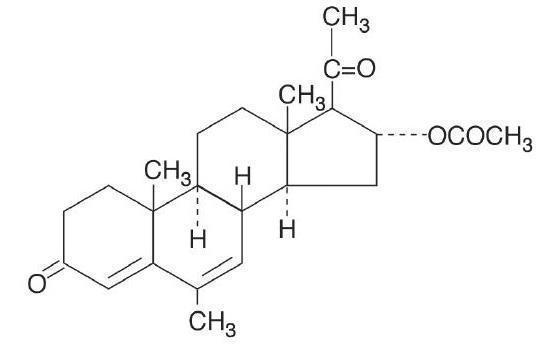
11 DESCRIPTIONMegestrol acetate oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Several investigators have reported on the appetite enhancing property of megestrol acetate and its possible use in cachexia. The precise mechanism by which megestrol ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Data on carcinogenesis were obtained from studies conducted in dogs, monkeys and rats treated with megestrol acetate at doses below the ...
-
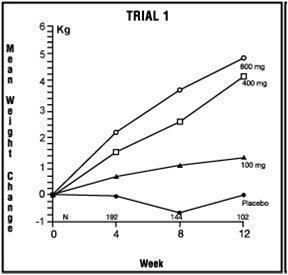
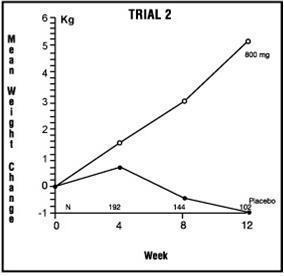
14 CLINICAL STUDIESThe efficacy of megestrol acetate oral suspension, 125 mg/mL, was based on two trials of megestrol acetate oral suspension (40 mg/mL). These two trials are described below. Trial 1 - One was a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Megestrol acetate oral suspension is a milky white, lemon-lime flavored oral suspension containing 125 mg per mL. Available in bottles of 150 mL (5 fl oz) NDC ...
-
17 PATIENT COUNSELING INFORMATION023The prescriber should inform the patient about the product differences to avoid overdosing or underdosing of megestrol acetate. The recommended adult dosage of megestrol acetate oral suspension ...
-
PRINCIPAL DISPLAY PANEL - 150 mL Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information