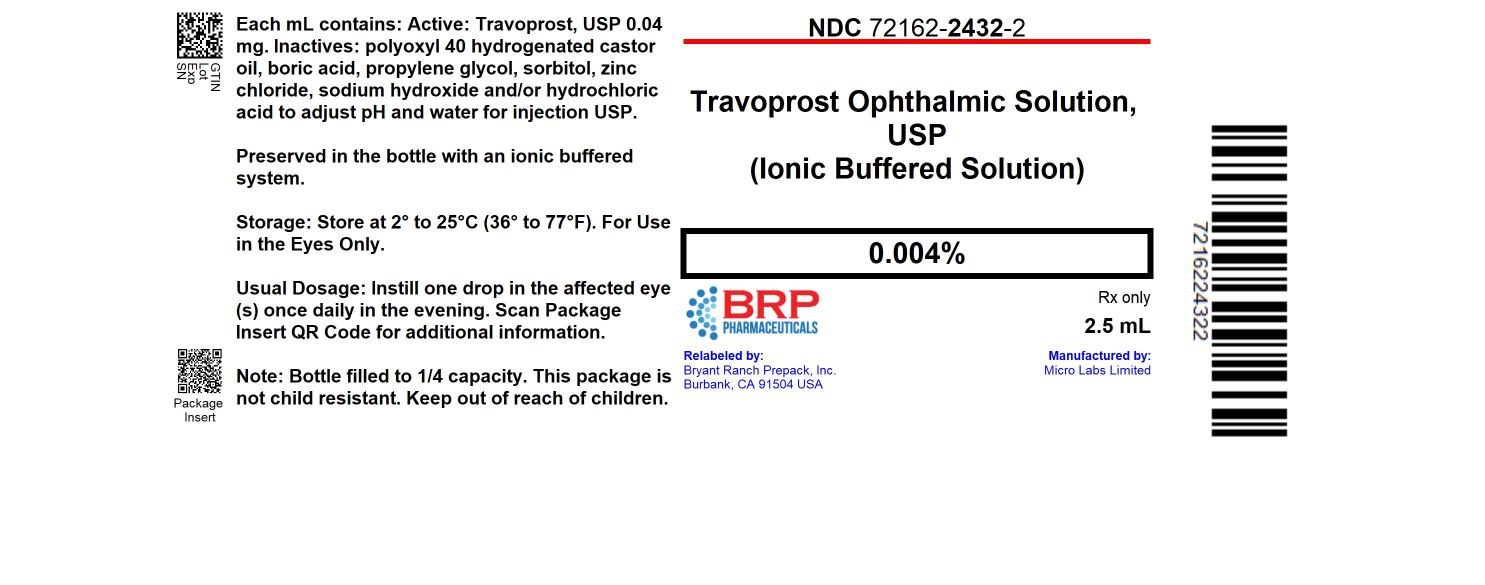
Label: TRAVOPROST OPHTHALMIC- travoprost ophthalmic solution solution
- NDC Code(s): 72162-2432-2, 72162-2432-4
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 42571-130
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRAVOPROST OPHTHALMIC SOLUTION (Ionic Buffered Solution) safely and effectively. See full prescribing information for TRAVOPROST ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETravoprost ophthalmic solution 0.004% (ionic buffered solution) is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage is one drop in the affected eye(s) once daily in the evening. Travoprost ophthalmic solution 0.004% (ionic buffered solution) should not be administered more than once daily ...
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing travoprost USP, 0.04 mg/mL.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Pigmentation - Travoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women to inform a drug-associated risk. In animal reproduction studies, subcutaneous (SC ...
-
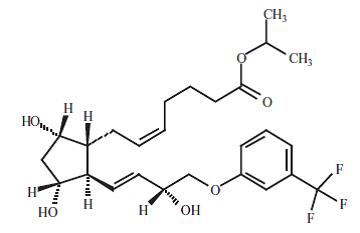
11 DESCRIPTIONTravoprost is a synthetic prostaglandin F analog. Its chemical name is [1R-[1α(Z),2β(1E,3R*),3α,5α]]-7-[3,5-Dihydroxy-2-[3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Travoprost free acid, a prostaglandin analog is a selective FP prostanoid receptor agonist which is believed to reduce IOP by increasing uveoscleral outflow. The exact ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year carcinogenicity studies in mice and rats at subcutaneous doses of 10, 30, or 100 mcg/kg/day did not show any evidence of ...
-
14 CLINICAL STUDIESIn clinical studies, patients with open-angle glaucoma or ocular hypertension and baseline pressure of 25 to 27 mmHg, who were treated with travoprost ophthalmic solution 0.004% or travoprost ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTravoprost Ophthalmic Solution USP, 0.004% (Ionic Buffered Solution) is a sterile, isotonic, buffered, preserved, aqueous solution of travoprost, USP (0.04 mg/mL) supplied in 3 piece open nozzle ...
-
17 PATIENT COUNSELING INFORMATIONPotential for Pigmentation - Advise the patient about the potential for increased brown pigmentation of the iris, which may be permanent. Inform the patient about the possibility of eyelid skin ...
-
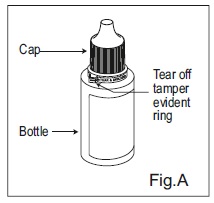
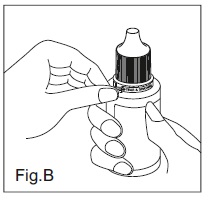
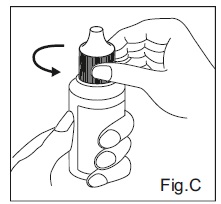
INSTRUCTIONS FOR USE:Travoprost Ophthalmic Solution USP, 0.004% (Ionic Buffered Solution) Before you use Travoprost Ophthalmic Solution USP, 0.004% (Ionic Buffered Solution) for the first time: 1. Check to ...
-
PRINCIPAL DISPLAY PANELTravoprost 0.004% Ophth Solution #2.5 - Extended Label
-
INGREDIENTS AND APPEARANCEProduct Information