Label: VAXCHORA- cholera vaccine, live, oral kit
- NDC Code(s): 50632-015-02
- Packager: Bavarian Nordic A/S
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VAXCHORA safely and effectively. See full prescribing information for VAXCHORA. VAXCHORA® (Cholera Vaccine, Live, Oral) Suspension ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera-affected areas. 1.1 ...
-
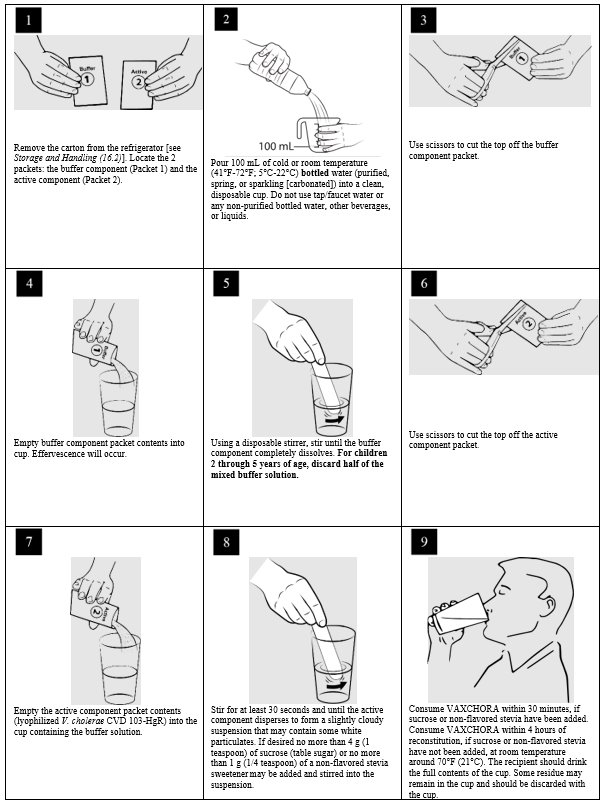
2 DOSAGE AND ADMINISTRATIONFor oral administration only. 2.1 Dose and Schedule - Administer a single oral dose of VAXCHORA a minimum of 10 days before potential exposure to cholera. The safety and effectiveness of ...
-
3 DOSAGE FORMS AND STRENGTHSVAXCHORA is a suspension for oral administration. Before reconstitution, each dose of VAXCHORA is supplied as a foil packet of buffer and an accompanying foil packet of the active component ...
-
4 CONTRAINDICATIONSDo not use in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Altered Immunocompetence - The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons [see Immunocompromised Individuals (8.6)]. 5.2 Shedding and ...
-
6 ADVERSE REACTIONS Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a vaccine cannot be directly compared to rates in the clinical trials ...
-
7 DRUG INTERACTIONS7.1 Food and Drink - Avoid food or drink for 60 minutes before and after vaccine administration [see Restrictions on Eating and Drinking (2.2)]. 7.2 Concomitant Vaccines or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - VAXCHORA is not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug. Clinical ...
-
11 DESCRIPTIONVAXCHORA (Cholera Vaccine, Live, Oral) is a live, attenuated bacterial vaccine suspension for oral administration containing the V. cholerae strain CVD 103-HgR. CVD 103-HgR was constructed from ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - VAXCHORA contains live attenuated cholera bacteria that replicate in the gastrointestinal tract of the recipient. Immune mechanisms conferring protection against ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - VAXCHORA has not been evaluated for the potential to cause carcinogenicity or genotoxicity, or to impair fertility.
-
14 CLINICAL STUDIES14.1 Efficacy Against V. cholerae Challenge - Study 2 was a randomized, double-blind, saline placebo-controlled V. cholerae challenge study conducted in the US. Subjects 18 through 45 years of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VAXCHORA is supplied as shown in Table 8. The contents of both packets are reconstituted with bottled water (purified, spring, or sparkling [carbonated]), to form one oral ...
-
17 PATIENT COUNSELING INFORMATIONPrior to administration of this vaccine, the health care professional should inform the individual of the following: • Advise vaccine recipients to exercise caution regarding food and water ...
-
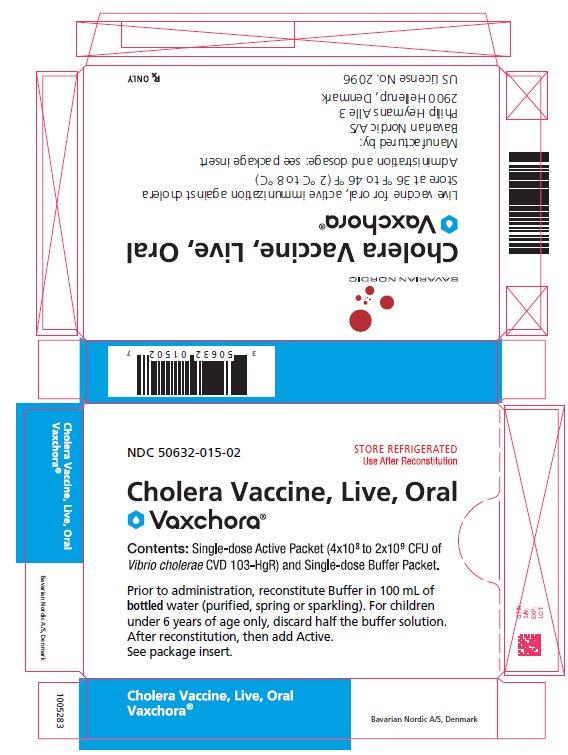
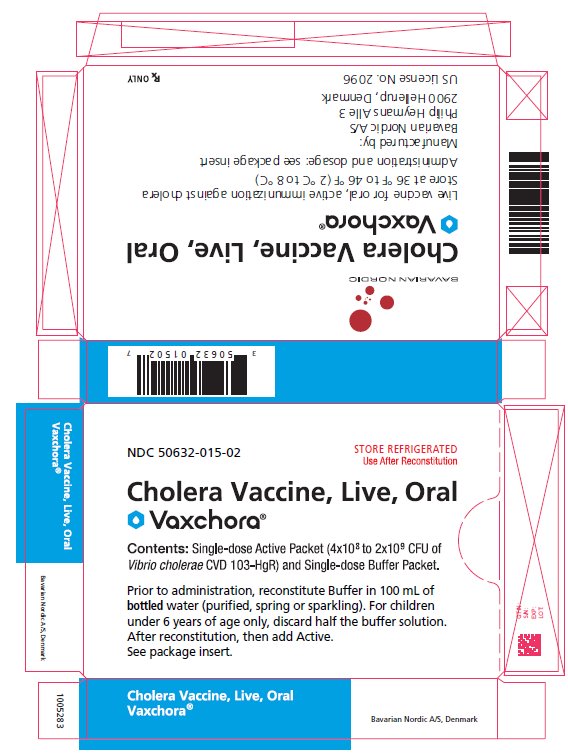
PRINCIPAL DISPLAY PANEL - Carton Carton - NDC 50632-015-02 - STORE REFRIGERATED - Use After Reconstitution - Cholera Vaccine, Live, Oral - Vaxchora® Contents: Single-dose Active Packet (4x108 to 2x109 CFU of - Vibrio cholerae ...
-
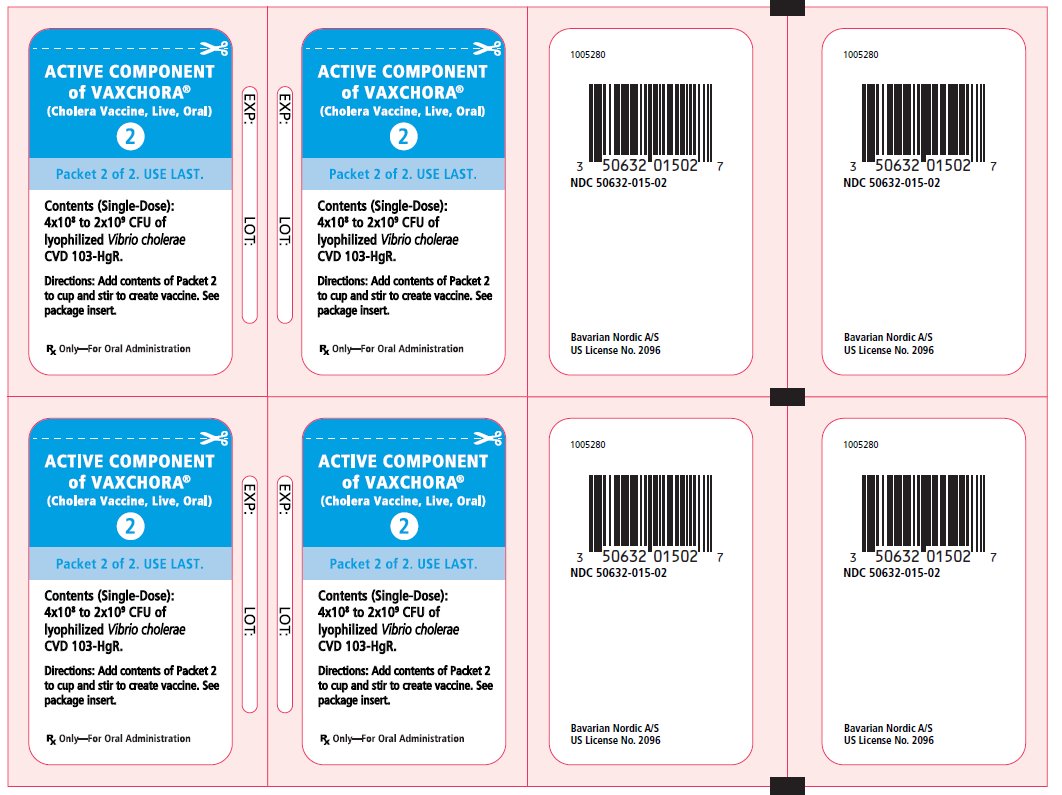
PRINCIPAL DISPLAY PANEL - Buffer and Active Packets BUFFER COMPONENT - OF VAXCHORA® (Cholera Vaccine, Live, Oral) 1 - Packet 1 of 2. USE FIRST. Contents (Single-Dose): Buffer - Directions: Add entire contents - of Packet 1 to 100 mL of ...
-
INGREDIENTS AND APPEARANCEProduct Information