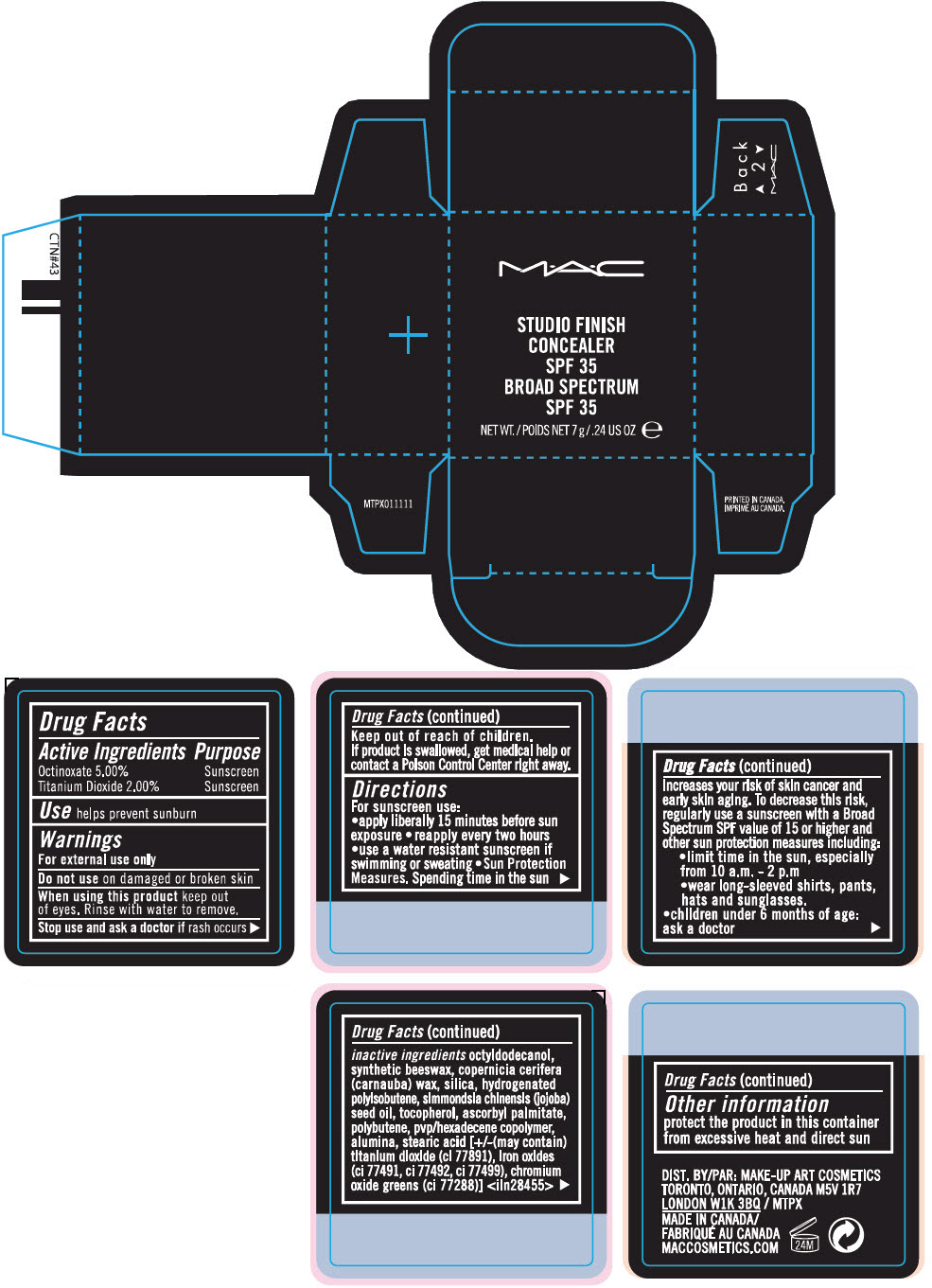
Label: STUDIO FINISH CONCEALER BROAD SPECTRUM SPF 35- octinoxate and titanium dioxide paste
- NDC Code(s): 40046-0078-1
- Packager: MAKEUP ART COSMETICS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m
- wear long-sleeved shirts, pants, hats and sunglasses.
- children under 6 months of age: ask a doctor
-
inactive ingredients
octyldodecanol, synthetic beeswax, copernicia cerifera (carnauba) wax, silica, hydrogenated polyisobutene, simmondsia chinensis (jojoba) seed oil, tocopherol, ascorbyl palmitate, polybutene, pvp/hexadecene copolymer, alumina, stearic acid [+/-(may contain) titanium dioxide (ci 77891), iron oxides (ci 77491, ci 77492, ci 77499), chromium oxide greens (ci 77288)] <iln28455>
- Other information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 7 g Jar Carton
-
INGREDIENTS AND APPEARANCE
STUDIO FINISH CONCEALER BROAD SPECTRUM SPF 35
octinoxate and titanium dioxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:40046-0078 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2 g in 100 g Inactive Ingredients Ingredient Name Strength OCTYLDODECANOL (UNII: 461N1O614Y) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) JOJOBA OIL (UNII: 724GKU717M) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) ALUMINUM OXIDE (UNII: LMI26O6933) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CHROMIC OXIDE (UNII: X5Z09SU859) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40046-0078-1 1 in 1 CARTON 08/08/2011 1 7 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/08/2011 Labeler - MAKEUP ART COSMETICS (010597206) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 202952982 manufacture(40046-0078) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 pack(40046-0078) , label(40046-0078)