Label: SANITIZING WIPES- benzalkonium chloride swab
- NDC Code(s): 72202-200-01, 72202-200-24, 72202-200-80
- Packager: Zogics, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
NDC 72202-200-01
2000 count
zogics Sanitizing Wipes
Reorder# Z2000 888.623.0088
Pre-saturated, multipurpose wipes for use in school, offices, fitness centers, grocery stores, healthcare settings and more.
Kills 99.9% of most common germs.
Gentle and effective alcohol-free formula that cleans and sanitizes.
Testured for thorough cleaning
Net Content: 2000 Wipes per Roll (8"X5.25")
Zogics, LLC Made in USA
Any Questions? We love to help: hello@zogicz.com or 888.623.0088

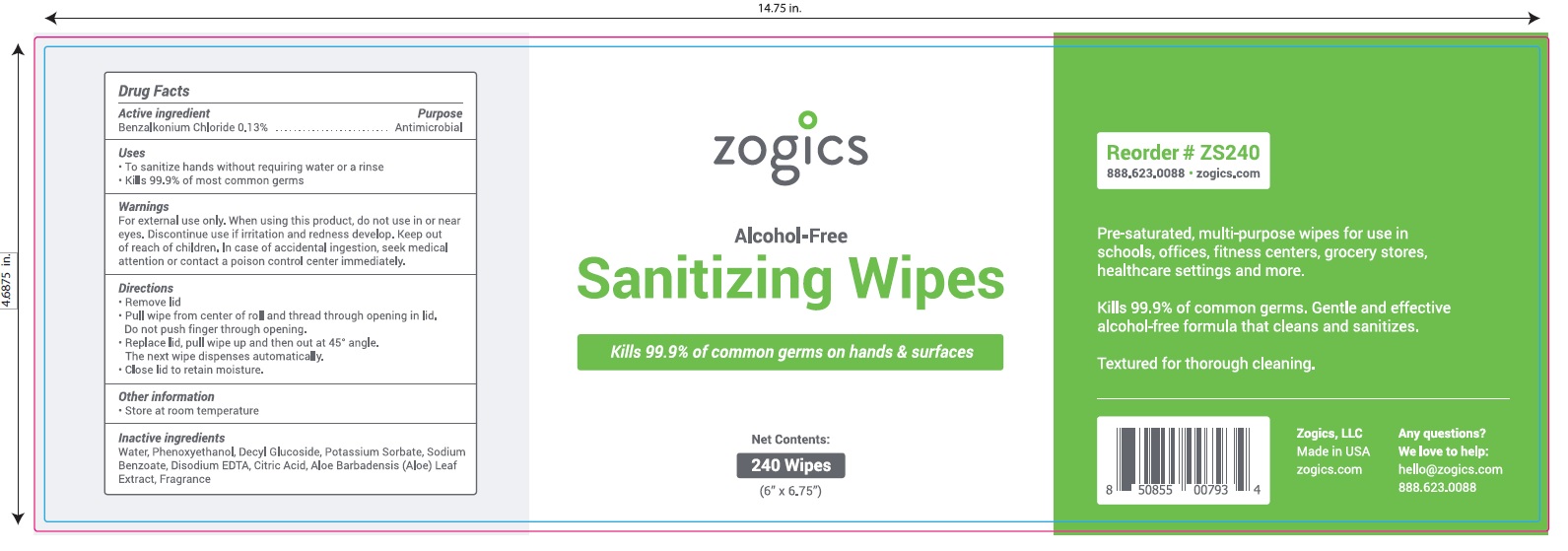
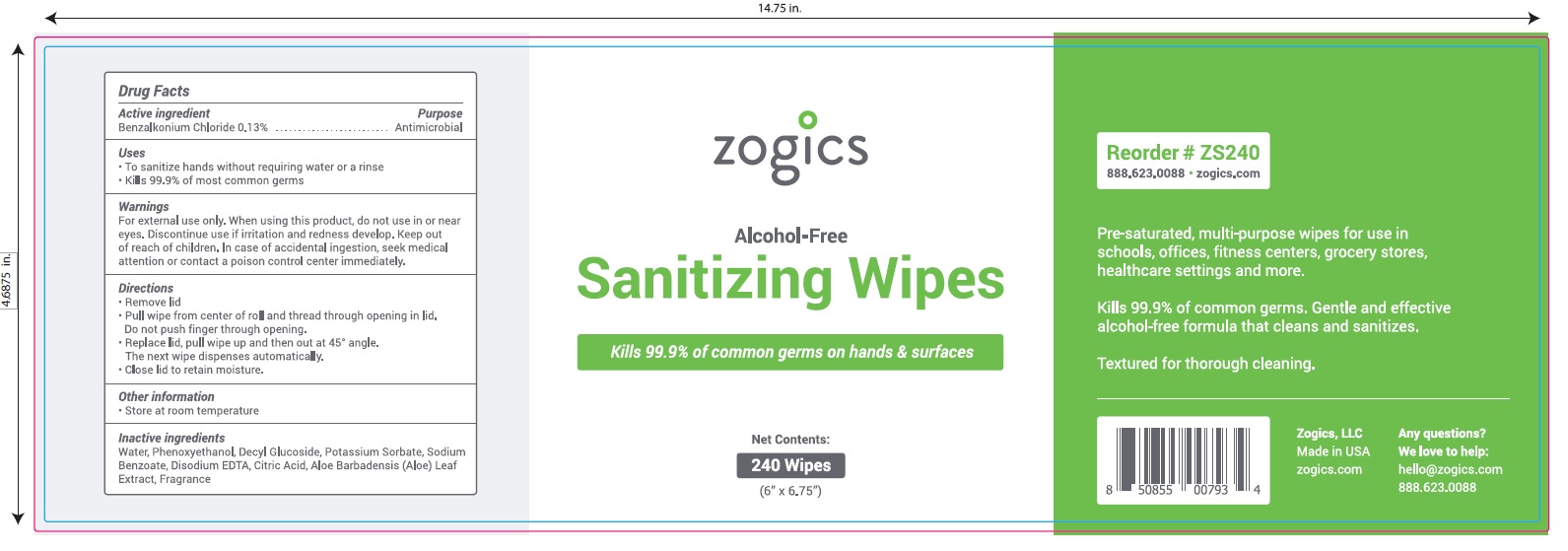
NDC 72202-200-24
zogics
Alcohol- Free
Sanitizing Wipes
Kills 99.9% of common germs on hands and surfaces.
Pre-saturated, multi-purpose wipes for use in schools, offices, fitness centers, grocery stores, healthcare settings and more.
Kills 99.9% of common germs.
Gentle and effective alcohol-free formula that cleans and sanitizes
Textured for thorough cleaning.
Net Contents: 240 Wipes (6" x 6.75")
Zogics, LLC Made in USA
Any Questions? We love to help: hello@zogicz.com or 888.623.0088
-
INGREDIENTS AND APPEARANCE
SANITIZING WIPES
benzalkonium chloride swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72202-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72202-200-01 2000 in 1 BAG 04/02/2018 1 1 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:72202-200-24 240 in 1 BAG 04/02/2018 2 1 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:72202-200-80 800 in 1 BAG 12/10/2024 3 1 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 04/02/2018 Labeler - Zogics, LLC (796321870) Registrant - Zogics, LLC (796321870) Establishment Name Address ID/FEI Business Operations Precare Corp. 117111327 manufacture(72202-200)