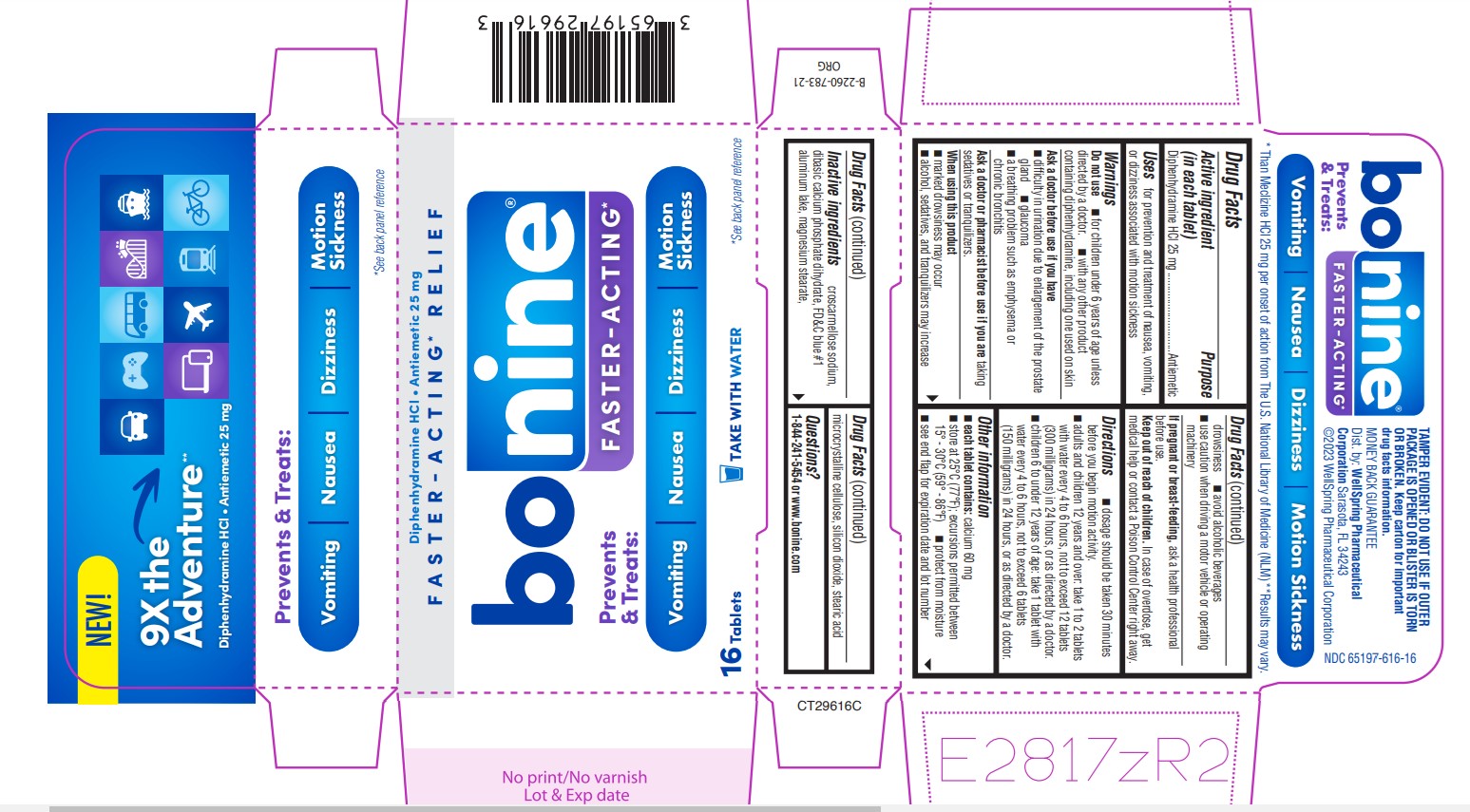
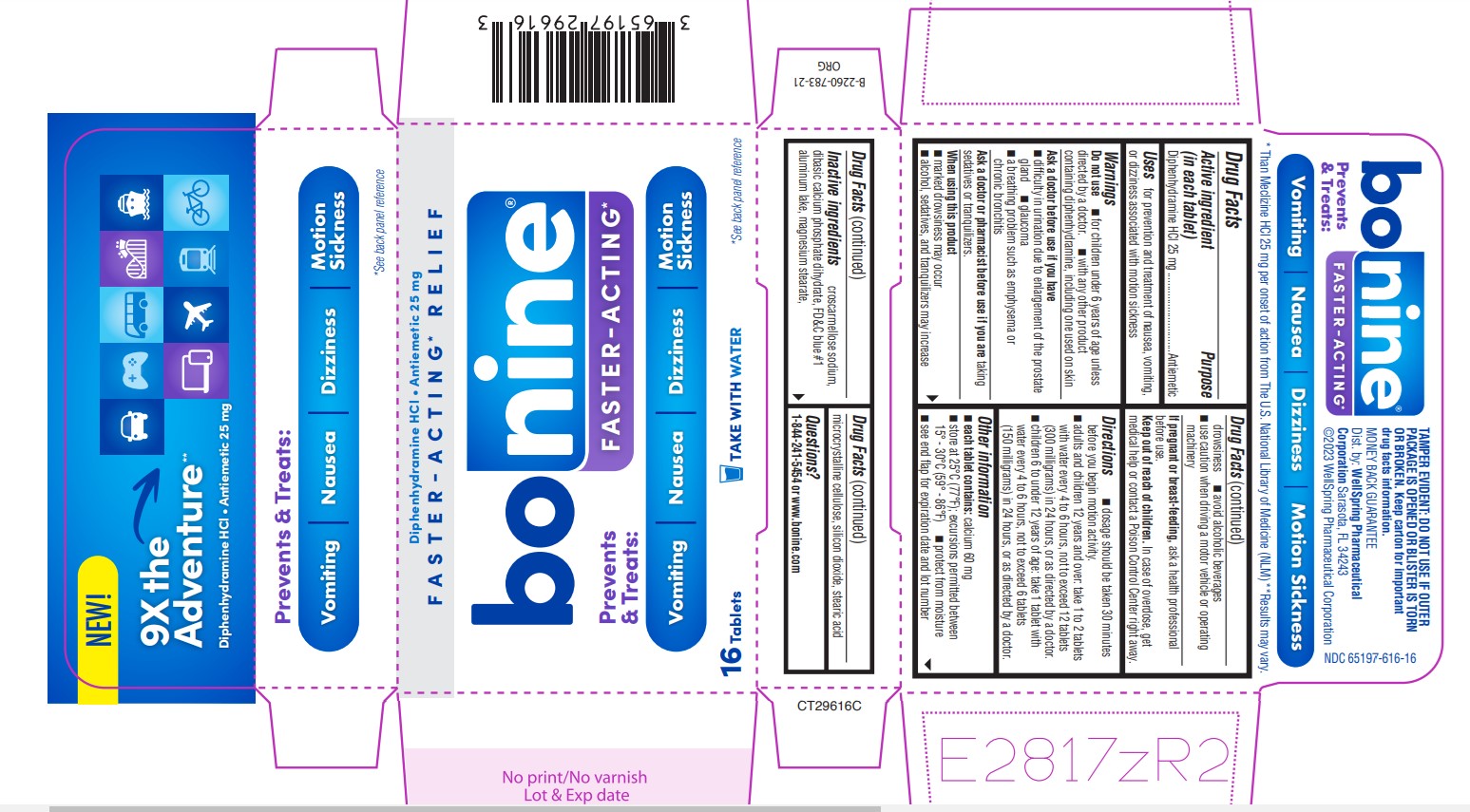
Label: BONINE FASTER-ACTING- diphenhydramine hcl tablet
- NDC Code(s): 65197-616-16
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
-
Purpose
Antiemetic
-
Uses
for prevention and treatment of nausea, vomiting, Uses or dizziness associated with motion sickness
-
Warnings
Do not use - ■ for children under 6 years of age unless - ■ Do not use with any other product containing diphenhydramine, including one used on skin - Ask a doctor before use if you have - ...
-
Directions
■ dosage should be taken 30 minutes before you begin motion activity. ■ adults and children 12 years and over: take 1 to 2 tablets with Water every 4 to 6 hours, not to exceed 12 tablets (300 ...
-
Other information■ Each tablet contains: Calcium 60mg - ■ Store at 25°C (77°F); excursions permitted between 15° - 30°C (59° - 86°F) ■ protect from moisture - ■ see end flap for expiration date and lot number
-
Inactive ingredients
Croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1, aluminum lake, magnesium stearate, microcrystalline cellulose, silicone dioxide, stearic acid
-
Questions?
1-844-241-5454 or www.bonine.com
-
SPL UNCLASSIFIED SECTIONTAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN. Keep Carton for important drug facts information. Distributed by: WellSpring - Pharmaceutical ...
-
PRINCIPAL DISPLAY PANEL 65197-616-16NEW - 9X the Adventure** Diphenhydramine HCl - Antiemetic 25 mg - Prevents & Treats: Vomiting • Nausea • Dizziness • Motion Sickness - *See back panel reference - * Than Meclizine HCL 25 mg per onset ...
-
INGREDIENTS AND APPEARANCEProduct Information