Label: FAMOTIDINE injection, solution
- NDC Code(s): 25021-753-02, 25021-754-04, 25021-754-20
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONsagent® Rx only
-
DESCRIPTION
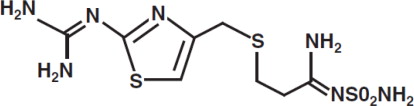
The active ingredient in Famotidine Injection, USP is a histamine H2-receptor antagonist. Famotidine is [1-Amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio] propylidene] sulfamide ...
-
CLINICAL PHARMACOLOGY IN ADULTS
GI Effects - Famotidine is a competitive inhibitor of histamine H2-receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric secretion. Both the ...
-
CLINICAL PHARMACOLOGY IN PEDIATRIC PATIENTS
Pharmacokinetics - Table 6 presents pharmacokinetic data from clinical trials and a published study in pediatric patients (<1 year of age; N=27) given famotidine IV 0.5 mg/kg and from published ...
-
INDICATIONS AND USAGE
Famotidine Injection, supplied as a concentrated solution for intravenous injection, is intended for intravenous use only. Famotidine Injection is indicated in some hospitalized patients with ...
-
CONTRAINDICATIONS
Hypersensitivity to any component of these products. Cross sensitivity in this class of compounds has been observed. Therefore, Famotidine Injection should not be administered to patients with a ...
-
WARNINGS
Famotidine Injection 4 mL and 20 mL multiple dose vials contain the preservative benzyl alcohol. There have been reports of fatal ‘gasping syndrome’ in neonates (children less than one month of ...
-
PRECAUTIONS
General - Symptomatic response to therapy with famotidine does not preclude the presence of gastric malignancy. Patients with Moderate or Severe Renal Insufficiency - Since CNS adverse ...
-
ADVERSE REACTIONS
The adverse reactions listed below have been reported during domestic and international clinical trials in approximately 2,500 patients. In those controlled clinical trials in which famotidine ...
-
OVERDOSAGE
The adverse reactions in overdose cases are similar to the adverse reactions encountered in normal clinical experience (see ADVERSE REACTIONS). Oral doses of up to 640 mg/day have been given to ...
-
DOSAGE AND ADMINISTRATION
In some hospitalized patients with pathological hypersecretory conditions or intractable ulcers, or in patients who are unable to take oral medication, Famotidine Injection may be administered ...
-
HOW SUPPLIED
FOR INTRAVENOUS USE ONLY - Famotidine Injection, USP, is supplied as follows: NDCFamotidine Injection, USP (Preservative-free)Package Factor - (10 mg per mL) 25021-753-02 - 20 mg per 2 mL ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-753-02 - Rx only - Famotidine Injection, USP - 20 mg per 2 mL - (10 mg per mL) For Intravenous Use Only After Dilution - 2 mL Single-Dose ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-754-04 - Rx only - Famotidine Injection, USP - 40 mg per 4 mL - (10 mg per mL) For Intravenous Use Only After Dilution - 4 mL Two-Dose ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-754-20 - Rx only - Famotidine Injection, USP - 200 mg per 20 mL - (10 mg per mL) For The Preparation of Intravenous Solutions - For ...
-
INGREDIENTS AND APPEARANCEProduct Information