Label: BROMFENAC solution/ drops
- NDC Code(s): 60219-1526-3
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BROMFENAC OPHTHALMIC SOLUTION, safely and effectively. See full prescribing information for BROMFENAC OPHTHALMIC ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBromfenac ophthalmic solution, 0.07% is indicated for the treatment of postoperative inflammation and reduction of ocular pain in patients who have undergone cataract surgery.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - One drop of bromfenac ophthalmic solution should be applied to the affected eye once daily beginning 1 day prior to cataract surgery, continued on the day of surgery, and ...
-
3 DOSAGE FORMS AND STRENGTHSTopical ophthalmic solution: bromfenac 0.07%.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Sulfite Allergic Reactions - Contains sodium sulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Treatment of rats at oral doses up to 0.9 mg/kg/day (systemic exposure 90 times the systemic exposure predicted from the recommended human ophthalmic dose [RHOD] assuming the ...
-
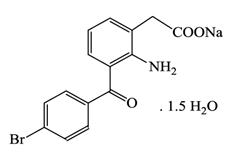
11 DESCRIPTIONBromfenac ophthalmic solution, 0.07% is a sterile, topical, nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Each mL of bromfenac ophthalmic solution contains 0.805 mg bromfenac ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) that has anti-inflammatory activity. The mechanism of its action is thought to be due to its ability to block ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies in rats and mice given oral doses of bromfenac up to 0.6 mg/kg/day (systemic exposure 30 times the ...
-
14 CLINICAL STUDIES14.1 Ocular Inflammation and Pain - Bromfenac 0.07% QD for the treatment of postoperative inflammation and reduction of ocular pain was evaluated in two multi-center, randomized, double-masked ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBromfenac ophthalmic solution is a clear, yellow solution and supplied in white LDPE opaque bottle with a LDPE white opaque nozzle and HDPE grey cap. Each mL contains bromfenac sodium ...
-
17 PATIENT COUNSELING INFORMATION17.1 Slowed or Delayed Healing - Advise patients of the possibility that slow or delayed healing may occur while using NSAIDs. 17.2 Sterility of Dropper Tip - Advise patients to replace ...
-
PRINCIPAL DISPLAY PANELNDC 60219-1526-3 - Bromfenac ophthalmic solution, 0.07% (3 mL) Rx only - Amneal Pharmaceuticals LLC
-
INGREDIENTS AND APPEARANCEProduct Information