Label: ENDOMETRIN- progesterone insert
- NDC Code(s): 55566-6500-1, 55566-6500-2, 55566-6500-3
- Packager: Ferring Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENDOMETRIN safely and effectively.
See full prescribing information for ENDOMETRIN.
ENDOMETRIN ® (progesterone)
Vaginal Insert 100 mg
Initial U.S. Approval: 2007INDICATIONS AND USAGE
ENDOMETRIN® is a progesterone indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART) treatment program for infertile women. (1)
DOSAGE AND ADMINISTRATION
The dose of ENDOMETRIN is 100 mg administered vaginally two or three times daily starting the day after oocyte retrieval and continuing for up to 10 weeks total duration. Efficacy in women 35 years of age and older has not been clearly established. The appropriate dose of ENDOMETRIN in this age group has not been determined. (2.1)
DOSAGE FORMS AND STRENGTHS
- 100 mg vaginal insert (3)
CONTRAINDICATIONS
- Previous allergic reactions to progesterone or any of the ingredients of ENDOMETRIN Vaginal Insert (4)
- Undiagnosed vaginal bleeding (4)
- Known missed abortion or ectopic pregnancy (4)
- Liver disease (4)
- Known or suspected malignancy of the breast or genital organs (4)
- Active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events (4)
WARNINGS AND PRECAUTIONS
- Life-threatening arterial or venous thromboembolic disorders may occur during hormone treatment, including treatment with ENDOMETRIN. Discontinue ENDOMETRIN if any of these are suspected. (5.1)
- Observe patients with a history of depression closely. Consider discontinuation if symptoms worsen. (5.2)
- ENDOMETRIN is not recommended for use with other vaginal products (such as antifungal products) as this may alter progesterone release and absorption from the vaginal insert. (5.3)
ADVERSE REACTIONS
The most common adverse reactions reported (greater than 2%) were post-oocyte retrieval pain, abdominal pain, nausea, and ovarian hyperstimulation syndrome. (6)
This leaflet summarizes the most important information about ENDOMETRIN. To report SUSPECTED ADVERSE REACTIONS, contact Ferring Pharmaceuticals Inc. at 1-888-FERRING (1-888-337-7464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular or Cerebrovascular Disorders
5.2 Depression
5.3 Use of Other Vaginal Products
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Expected Adverse Reaction Profile Seen with Progesterone
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Luteal Supplementation During Assisted Reproductive Treatment Study
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Vaginal Bleeding
17.2 Common Adverse Reactions with Progesterone
17.3 Coadministration of Other Vaginal Products
17.4 FDA-Approved Patient Labeling
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
The dose of ENDOMETRIN is 100 mg administered vaginally two or three times daily starting the day after oocyte retrieval and continuing for up to 10 weeks total duration. Efficacy in women 35 years of age and older has not been clearly established. The appropriate dose of ENDOMETRIN in this age group has not been determined.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ENDOMETRIN is contraindicated in individuals with any of the following conditions:
- Previous allergic reactions to progesterone or any of the ingredients of ENDOMETRIN [see Description (11)]
- Undiagnosed vaginal bleeding
- Known missed abortion or ectopic pregnancy
- Liver disease
- Known or suspected malignancy of the breast or genital organs
- Active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular or Cerebrovascular Disorders
The physician should be alert to earliest signs of myocardial infarction, cerebrovascular disorders, arterial or venous thromboembolism (venous thromboembolism or pulmonary embolism), thrombophlebitis, or retinal thrombosis. ENDOMETRIN should be discontinued if any of these are suspected.
5.2 Depression
Patients with a history of depression need to be closely observed. Consider discontinuation if symptoms worsen.
5.3 Use of Other Vaginal Products
ENDOMETRIN should not be recommended for use with other vaginal products (such as antifungal products) as this may alter progesterone release and absorption from the vaginal insert [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data reflect exposure to ENDOMETRIN in 808 infertile women (74.9% White, 10.3% Hispanic, 5.4% Black, 5% Asian, and 4.6% Other) in a single Assisted Reproductive Technology 10 week clinical study conducted in the U.S. ENDOMETRIN was studied at doses of 100 mg twice daily and 100 mg three times daily. The adverse reactions that occurred at a rate greater than or equal to 2% in either ENDOMETRIN group are summarized in Table 1.
Table 1: Number and Frequency of Reported Adverse Reactions in Women Treated with ENDOMETRIN in an Assisted Reproductive Technology Study Body System ENDOMETRIN
100 mg twice daily
(N=404)ENDOMETRIN
100 mg three times daily
(N=404)Preferred Term Gastrointestinal Disorders Abdominal pain 50 (12%) 50 (12%) Nausea 32 (8%) 29 (7%) Abdominal distension 18 (4%) 17 (4%) Constipation 9 (2%) 14 (3%) Vomiting 13 (3%) 9 (2%) General Disorders and Administration Site Conditions Fatigue 7 (2%) 12 (3%) Infections and Infestations Urinary tract infection 9 (2%) 4 (1%) Injury, Poisoning and Procedural Complications Post-oocyte retrieval pain 115 (28%) 102 (25%) Nervous System Disorders Headache 15 (4%) 13 (3%) Reproductive System and Breast Disorders Ovarian hyperstimulation syndrome 30 (7%) 27 (7%) Uterine spasm 15 (4%) 11 (3%) Vaginal bleeding 13 (3%) 14 (3%) Other less common reported adverse reactions included vaginal irritation, itching, burning, discomfort, urticaria, and peripheral edema.
-
7 DRUG INTERACTIONS
No formal drug-drug interaction studies have been conducted for ENDOMETRIN. Drugs known to induce the hepatic cytochrome-P450-3A4 system (such as rifampin, carbamazepine) may increase the elimination of progesterone.
The effect of concomitant vaginal products on the exposure of progesterone from ENDOMETRIN has not been assessed. ENDOMETRIN is not recommended for use with other vaginal products (such as antifungal products) as this may alter progesterone release and absorption from the vaginal insert [see Warnings and Precautions (5.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
ENDOMETRIN has been used to support embryo implantation and maintain clinical pregnancy in one clinical study. The live birth outcomes of these pregnancies were as follows:
- Among the 404 subjects treated with ENDOMETRIN twice daily, 143 subjects had live births consisting of 85 singletons, 56 twins, and 2 triplets. In this treatment group, 13 subjects had a spontaneous abortion, 1 subject had an ectopic pregnancy, and 7 subjects reported fetal birth defects (3.4% based on 203 livebirths).
- Among the 404 subjects treated with ENDOMETRIN three times daily, 155 subjects had livebirths consisting of 91 singletons, 60 twins, and 4 triplets. In this treatment group, 22 subjects had a spontaneous abortion, 4 subjects had an ectopic pregnancy, and 7 subjects reported fetal birth defects (3.1% based on 223 livebirths).
Birth defects reported in the ENDOMETRIN twice daily group included: one fetus with a cleft palate and intrauterine growth retardation, one fetus with spina bifida, three fetuses with congenital heart defects, one fetus with an umbilical hernia, and one fetus with an intestinal anomaly.
Birth defects reported in the ENDOMETRIN three times daily group included: one fetus with an esophageal fistula, one fetus with hypospadias and an underdeveloped right ear, one fetus with Down Syndrome and an atrial septal defect, one fetus with congenital heart anomalies, one fetus with DiGeorge's syndrome, one fetus with a hand deformity, and one fetus with cleft palate.
For additional information on the pharmacology of ENDOMETRIN and pregnancy outcome information [ see Clinical Pharmacology (12) and Clinical Studies Sections (14)].
8.3 Nursing Mothers
Detectable amounts of progesterone have been identified in the milk of nursing mothers. The effect of this on the nursing infant has not been determined.
- 10 OVERDOSAGE
-
11 DESCRIPTION
ENDOMETRIN (progesterone) Vaginal Insert contains micronized progesterone. ENDOMETRIN is supplied with polyethylene vaginal applicators.
The active ingredient, progesterone, is present in 100 mg amount along with other excipients. The chemical name for progesterone is pregn-4-ene-3,20-dione. It has an empirical formula of C21H30O2 and a molecular weight of 314.5. Progesterone exists in two polymorphic forms. The form used in ENDOMETRIN, the alpha-form, has a melting point of 127-131°C.
The structural formula is:
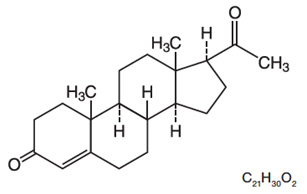
Each ENDOMETRIN Vaginal Insert delivers 100 mg of progesterone in a base containing lactose monohydrate, polyvinylpyrrolidone, adipic acid, sodium bicarbonate, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and colloidal silicon dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal gland. In the presence of adequate estrogen, progesterone transforms a proliferative endometrium into a secretory endometrium. Progesterone is necessary to increase endometrial receptivity for implantation of an embryo. Once an embryo is implanted, progesterone acts to maintain a pregnancy.
12.3 Pharmacokinetics
Absorption
Progesterone serum concentrations increased following the administration of the ENDOMETRIN Vaginal Insert in 12 healthy pre-menopausal females. On single dosing, the mean Cmax was 17.0 ng/mL in the ENDOMETRIN twice daily group and 19.8 ng/mL in the ENDOMETRIN three times daily group. On multiple dosing, steady-state concentrations were attained within approximately 1 day after initiation of treatment with ENDOMETRIN. Both ENDOMETRIN regimens provided average serum concentrations of progesterone exceeding 10 ng/mL on Day 5. The pharmacokinetic results are summarized in Table 2.
Table 2: Mean (±Standard Deviation) Serum Progesterone Pharmacokinetic Parameters Pharmacokinetic Parameter (unit) ENDOMETRIN 100 mg
twice daily (N=6)ENDOMETRIN 100 mg
three times daily (N=6)Cmax Maximum progesterone serum concentration. Tmax Time to maximum progesterone serum concentration. Cavg Average progesterone serum concentration. AUC0-24 Area under the drug concentration versus time curve from 0-24 hours post dose. Cmin Minimum progesterone serum concentration. Single Dosing Cmax (ng/mL) 17.0 ± 6.5 19.8 ± 7.2 Tmax (hr) 24.0 ± 0.0 17.3 ± 7.4 AUC0-24
(ng∙hr/mL)217 ± 113 284 ± 143 Day 5 of Multiple Dosing Cmax (ng/mL) 18.5 ± 5.5 24.1 ± 5.6 Tmax (hr) 18.0 ± 9.4 18.0 ± 9.4 Cmin (ng/mL) 8.9 ± 4.5 10.9 ± 6.7 Cavg (ng/ml) 14.0 ± 4.8 15.9 ± 4.3 AUC0-24
(ng∙hr/mL)327 ± 127 436 ± 106 Distribution
Progesterone is approximately 96% to 99% bound to serum proteins, primarily to serum albumin and corticosteroid binding globulin.
Metabolism
Progesterone is metabolized primarily by the liver, largely to pregnanediols and pregnanolones. Pregnanediols and pregnanolones are conjugated in the liver to glucuronide and sulfate metabolites. Progesterone metabolites that are excreted in the bile may be deconjugated and may be further metabolized in the gut via reduction, dehydroxylation, and epimerization.
Excretion
Progesterone undergoes renal and biliary elimination. Following injection of labeled progesterone, 50-60% of the excretion of metabolites occurs via the kidney; approximately 10% occurs via the bile and feces. Overall recovery of the labeled material accounts for 70% of an administered dose. Only a small portion of unchanged progesterone is excreted in the bile.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Luteal Supplementation During Assisted Reproductive Treatment Study
A randomized, open-label, active-controlled study evaluated the efficacy of 10 weeks of treatment with two different daily dosing regimens of ENDOMETRIN (100 mg twice daily and 100 mg three times daily) for support of implantation and early pregnancy in infertile women participating in an Assisted Reproductive Technology treatment program. Efficacy was assessed on the endpoint of ongoing pregnancies, defined as the presence of at least one fetal heartbeat seen on ultrasound at 6 weeks post-embryo transfer. The study randomized to ENDOMETRIN 808 infertile women (74.9% White; 10.3% Hispanic, 5.4% Black, 5% Asian, and 4.6% Other) between 19 and 42 years of age (mean age 33) who had a body mass index <34 kg/m2 at screening.
The ongoing pregnancy rates for subjects treated with both dosing regimens of ENDOMETRIN were non-inferior (lower bounds of the 95% confidence interval of the difference between ENDOMETRIN and the active comparator excluded a difference greater than 10%) to the ongoing pregnancy rate for subjects treated with the active comparator. The results of this study are shown in Table 3.
Table 3: Ongoing Pregnancy Rates* in Patients Receiving ENDOMETRIN for Luteal Supplementation and Early Pregnancy While in an Assisted Reproductive Technology Treatment Program ENDOMETRIN
100 mg twice dailyENDOMETRIN
100 mg three times daily- *
- Ongoing pregnancy defined as the presence of at least one fetal heartbeat seen on ultrasound at 6 weeks post-embryo transfer.
Number of subjects 404 404 Ongoing pregnancy: n (%) 156 (39%) 171 (42%) 95% Confidence Interval of pregnancy rate [33.8, 43.6] [37.5, 47.3] Pregnancy rate percentage difference between ENDOMETRIN and comparator -3.6% 0.1% 95% Confidence Interval for difference vs comparator [-10.3, 3.2] [-6.7, 6.9] Subjects participating in the study were stratified at randomization by age and ovarian reserve (as measured by serum FSH levels). The ongoing pregnancy rates for these subgroups are shown in Table 4.
Table 4: Ongoing Pregnancy Rates in Age- and Ovarian Reserve-Defined Subgroups Receiving ENDOMETRIN for Luteal Supplementation and Early Pregnancy While in an Assisted Reproductive Technology Treatment Program ENDOMETRIN
100 mg twice dailyENDOMETRIN
100 mg three times dailySubjects age < 35 years (N) 247 247 Ongoing pregnancy: n (%) 111 (45%) 117 (47%) Pregnancy rate percentage difference between ENDOMETRIN and comparator 0.5% 2.9% 95% Confidence Interval for difference vs. comparator [-8.3, 9.3] [-5.9, 11.7] Subjects 35-42 years of age (N) 157 157 Ongoing pregnancy: n (%) 45 (28%) 54 (34%) Pregnancy rate percentage difference between ENDOMETRIN and comparator -10.1% -4.4% 95% Confidence Interval for difference vs. comparator [-20.3, 0.3] [-14.9, 6.3] Subjects with FSH < 10 IU/L (N) 350 347 Ongoing pregnancy: n (%) 140 (40%) 150 (43%) Pregnancy rate percentage difference between ENDOMETRIN and comparator -2.0% 1.2% 95% Confidence Interval for difference vs. comparator [-9.3, 5.3] [-6.1, 8.5] Subjects with FSH between 10 and 15 IU/L (N) 46 51 Ongoing pregnancy: n (%) 16 (35%) 20 (39%) Pregnancy rate percentage difference between ENDOMETRIN and comparator -12.2% -7.7% 95% Confidence Interval for difference vs. comparator [-31.0, 7.7] [-26.6, 11.6] In subjects under the age of 35 or with serum FSH levels less than 10 IU/L, results from both dosing regimens were non-inferior to the results from the comparator with respect to ongoing pregnancy rates. In women age 35 and older and in women with serum FSH levels between 10 and 15 IU/L, the results with respect to ongoing pregnancy rates for both dosing regimens of ENDOMETRIN did not reach the criteria for non-inferiority.
Subjects who became pregnant received study medication for a total of 10 weeks. Patients over 34 kg/m2 were not studied. The efficacy of ENDOMETRIN in this patient group is unknown.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each ENDOMETRIN Vaginal Insert is a white to off-white oblong-shaped insert debossed with "FPI" on one side and "100" on the other side. Each ENDOMETRIN (progesterone) Vaginal Insert, 100 mg, is packed individually in a sealed foil pouch. These pouches are available in cartons packed:
- 21 vaginal inserts with 21 disposable vaginal applicators (NDC 55566-6500-3)
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.4).
17.1 Vaginal Bleeding
Inform patients of the importance of reporting irregular vaginal bleeding to their doctor as soon as possible.
17.2 Common Adverse Reactions with Progesterone
Inform patients of the possible side effects of progesterone therapy such as headaches, breast tenderness, bloating, mood swings, irritability, and drowsiness.
-
PATIENT PACKAGE INSERT
IMPORTANT: For Vaginal Use Only.
Read the patient information that comes with ENDOMETRIN ® (progesterone) before you start to use it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment. Your doctor may do a physical exam before prescribing ENDOMETRIN.
What is ENDOMETRIN?
ENDOMETRIN is a vaginal insert that contains the hormone progesterone. ENDOMETRIN is for women who need extra progesterone while undergoing treatment in an Assisted Reproductive Technology (ART) program.
Progesterone is one of the hormones essential for helping you to become and to stay pregnant. If you are undergoing ART treatment, your doctor may prescribe ENDOMETRIN to provide the progesterone your body needs.
Who should not use ENDOMETRIN?
Do not use ENDOMETRIN if you:
- Are allergic to anything in ENDOMETRIN. See the end of this leaflet for a complete list of ingredients.
- Have unusual vaginal bleeding that has not been evaluated by a doctor.
- Currently have or have had liver problems or cancer of the breast or genital organs.
- Have or have had blood clots in the legs, lungs, eyes, or elsewhere in your body.
ENDOMETRIN may not be right for you. Before starting ENDOMETRIN, tell your doctor about all your health problems.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vaginal products, vitamins, and herbal supplements.
Some medicines may affect ENDOMETRIN.
Know what medicines you take. Keep a list of your medicines to show to the doctor and pharmacist.
How should I use ENDOMETRIN?
- Use ENDOMETRIN exactly as prescribed. The usual dose of ENDOMETRIN is one insert placed in your vagina 2 to 3 times a day for up to a total of 10 weeks, unless your healthcare provider advises otherwise.
- Place an ENDOMETRIN insert in your vagina with the disposable applicator provided.
Follow the steps below:
- Unwrap the applicator. Do not use if the contents or packaging are visibly damaged.
- Put one insert in the space provided at the end of the applicator. The insert should fit snugly and not fall out.
- Place applicator with the insert into the vagina while you are standing, sitting, or when lying on your back with your knees bent. Gently place the thin end of the applicator well into the vagina.
- Push the plunger to release the insert.
- Remove the applicator and throw it away in the trash.
Other information for using ENDOMETRIN
- If you forget a dose of ENDOMETRIN, take the dose as soon as you remember, but do not use more than your daily dose.
- Call your doctor if you use too much ENDOMETRIN.
- Do not use any other vaginal products when you are using ENDOMETRIN.
What are the possible side effects of ENDOMETRIN?
Common side effects seen with ART and ENDOMETRIN included pelvic pain after surgery, abdominal pain, nausea, and swollen ovaries (ovarian hyperstimulation syndrome).
Other reported side effects included abdominal bloating, headache, urinary infections, uterine cramping, constipation, vomiting, tiredness, and vaginal bleeding.
Vaginal products with progesterone may also cause vaginal irritation, burning, and discharge.
Serious Risks of Progesterone
Progesterone can increase your chance of getting blood clots. Blood clots can be serious and lead to death.
Serious blood clots include those in the:
- legs (thrombophlebitis)
- lungs (pulmonary embolus)
- eyes (blindness)
- heart (heart attack)
- brain (stroke)
Call your doctor or get medical help right away if you have:
- persistent pain in the lower leg (calf)
- sudden shortness of breath
- coughing up blood
- sudden blindness, partial or complete
- severe chest pain
- sudden, severe headache, vomiting, dizziness, or fainting
- weakness in an arm or leg, or trouble speaking
- yellowing of the skin and/or white of the eyes indicating possible liver problem
Other risks of progesterone use include:
- headache
- breast tenderness
- bloating or fluid retention
- mood swings and depression
- irritability
- drowsiness
Call your doctor immediately if you have abnormal vaginal bleeding.
These are not all the side effects with ENDOMETRIN. Ask your doctor or pharmacist for more information.
How should I store ENDOMETRIN?
- Store ENDOMETRIN at room temperature, 20 - 25°C (68 - 77°F); excursions permitted between 15 - 30°C (59 - 86°F).
- Do not use ENDOMETRIN after the expiration date that is printed on the carton.
- Keep ENDOMETRIN and all medicines out of the reach of children.
General information about ENDOMETRIN
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use ENDOMETRIN for a condition for which it was not prescribed. Do not give ENDOMETRIN to other women, even if they have the same condition as you do. It may harm them.
This leaflet summarizes the most important information about ENDOMETRIN. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ENDOMETRIN that was written for healthcare professionals. For more information call Ferring Pharmaceuticals Inc. at 1-(888)-FERRING or 1-(888)-337-7464.
What are the ingredients in ENDOMETRIN?
Active Ingredient: progesterone
Inactive Ingredients: lactose monohydrate, polyvinylpyrrolidone, adipic acid, sodium bicarbonate, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and colloidal silicon dioxide
MANUFACTURED FOR:
FERRING PHARMACEUTICALS INC.
PARSIPPANY, NJ 070546485-03
Revised: 01/2018 -
PRINCIPAL DISPLAY PANEL - 100 mg Carton
NDC 55566-6500-3
Endometrin®
(progesterone) Vaginal Insert 100mgContents:
21 vaginal inserts with 21 disposable vaginal applicators
Each insert contains 100 mg progesterone, USPFOR VAGINAL USE ONLY
Rx only
Usual Dosage:
See enclosed package insert for dosage and
complete Prescribing Information.This carton contains instructions intended
for the patient.FERRING
PHARMACEUTICALS
-
INGREDIENTS AND APPEARANCE
ENDOMETRIN
progesterone insertProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55566-6500 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 100 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) ADIPIC ACID (UNII: 76A0JE0FKJ) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score Shape OVAL (Oblong) Size Flavor Imprint Code FPI;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55566-6500-3 1 in 1 CARTON 06/21/2007 1 NDC:55566-6500-2 21 in 1 CARTON 1 NDC:55566-6500-1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022057 06/21/2007 Labeler - Ferring Pharmaceuticals Inc. (103722955) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 pack(55566-6500) Establishment Name Address ID/FEI Business Operations QPharma 508638848 manufacture(55566-6500) , pack(55566-6500) Establishment Name Address ID/FEI Business Operations Pharmaceutics International Inc. 878265586 manufacture(55566-6500) Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company LLC 618054084 API MANUFACTURE(55566-6500)

