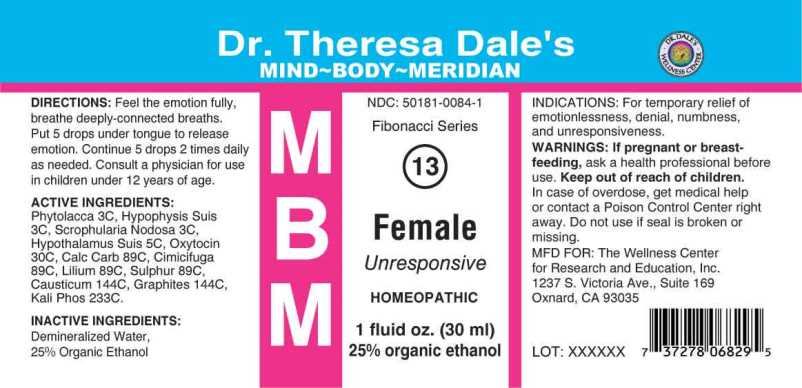
Label: MBM 13 FEMALE (phytolacca decandra, hypophysis (suis), scrophularia nodosa, hypothalamus- suis, calcarea carbonica, cimicifuga racemosa, lilium tigrinum, sulphur, causticum, graphites, kali phosphoricum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 50181-0084-1 - Packager: The Wellness Center for Research and Education, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT:
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY
-
INGREDIENTS AND APPEARANCE
MBM 13 FEMALE
phytolacca decandra, hypophysis (suis), scrophularia nodosa, hypothalamus (suis), calcarea carbonica, cimicifuga racemosa, lilium tigrinum, sulphur, causticum, graphites, kali phosphoricum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50181-0084 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 3 [hp_C] in 1 mL SUS SCROFA PITUITARY GLAND (UNII: L0PFEMQ1DT) (SUS SCROFA PITUITARY GLAND - UNII:L0PFEMQ1DT) SUS SCROFA PITUITARY GLAND 3 [hp_C] in 1 mL SCROPHULARIA NODOSA WHOLE (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA WHOLE 3 [hp_C] in 1 mL BOS TAURUS HYPOTHALAMUS (UNII: S6G2NLH4Y7) (BOS TAURUS HYPOTHALAMUS - UNII:S6G2NLH4Y7) BOS TAURUS HYPOTHALAMUS 5 [hp_C] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 89 [hp_C] in 1 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 89 [hp_C] in 1 mL LILIUM LANCIFOLIUM WHOLE FLOWERING (UNII: X67Z2963PI) (LILIUM LANCIFOLIUM WHOLE FLOWERING - UNII:X67Z2963PI) LILIUM LANCIFOLIUM WHOLE FLOWERING 89 [hp_C] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 89 [hp_C] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 144 [hp_C] in 1 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 144 [hp_C] in 1 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 233 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50181-0084-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 05/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/03/2022 Labeler - The Wellness Center for Research and Education, Inc. (832363993) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50181-0084) , api manufacture(50181-0084) , label(50181-0084) , pack(50181-0084)