Label: SENNA LAXATIVE- sennosides tablet
- NDC Code(s): 41163-775-10
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
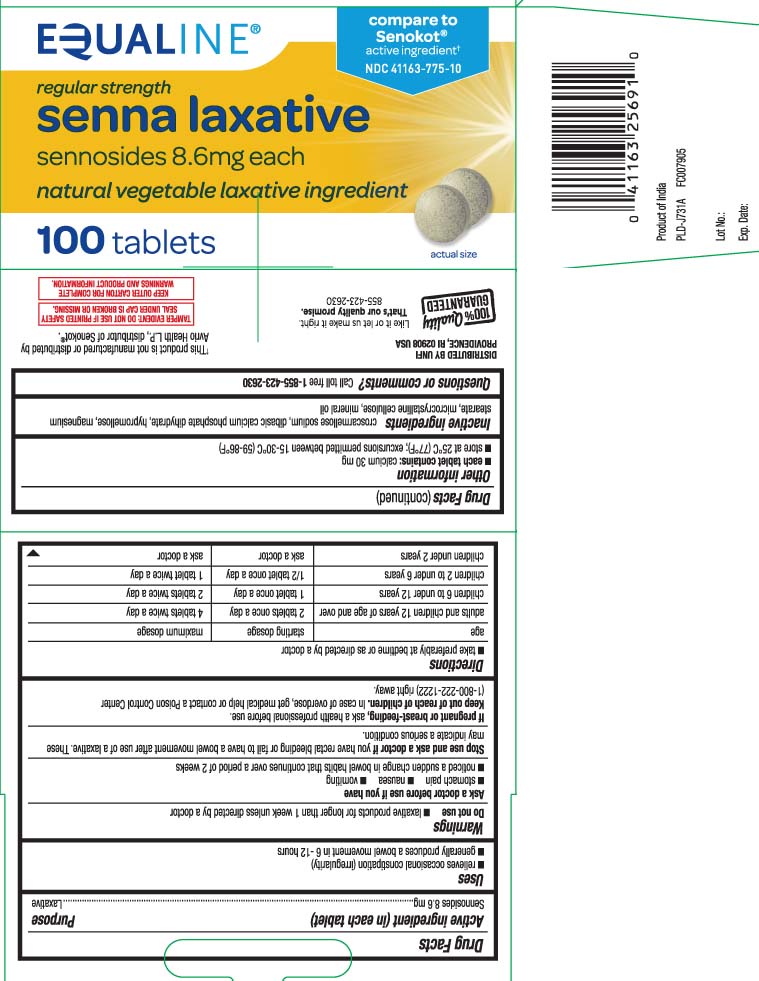
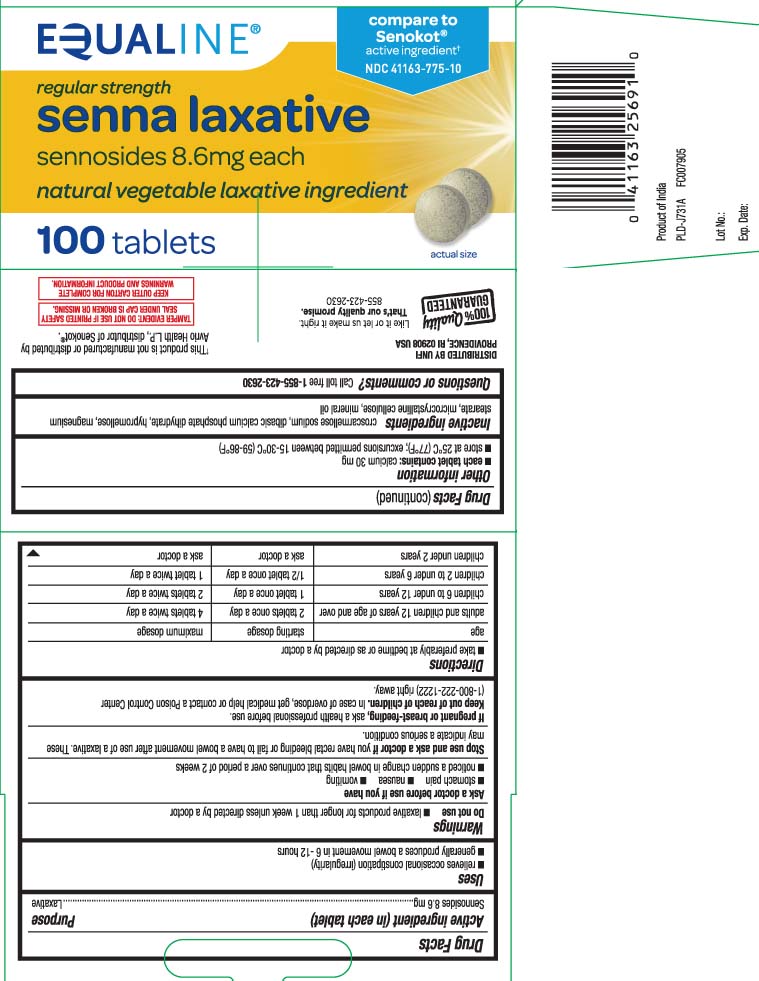
Active ingredient (in each tablet)Sennosides 8.6 mg
-
PurposeLaxative
-
Usesrelieves occasional constipation (irregularity) generally produces bowel movement in 6-12 hours
-
WarningsDo not use - laxative products for longer than 1 week unless directed by a doctor - Ask a doctor before use if you have - stomach pain - nausea - vomiting - noticed a sudden change in bowel habits ...
-
Directionstake preferably at bedtime or as directed by a doctor - agestarting dosage maximum dosage - adults and children 12 years of age and over2 tablets once a day4 tablets twice a ...
-
Other informationeach tablet contains: calcium 30 mg - store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF)
-
Inactive ingredientscroscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil
-
Questions or comments?Call toll free 1-855-423-2630
-
Principal Display Panelcompare to Senokot® active ingredient† regular strength - senna laxative - sennosides 8.6 mg each - natural vegetable laxative ingredient - tablets - †This product is not manufactured or distributed by ...
-
Product LabelingEQUALINE Senna Laxative
-
INGREDIENTS AND APPEARANCEProduct Information