Label: YARGESA- miglustat capsule
- NDC Code(s): 42799-709-01, 42799-709-15
- Packager: Edenbridge Pharmaceuticals LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 9, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use YARGESA safely and effectively. See full prescribing information for YARGESA. YARGESA (miglustat) capsules, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Type 1 Gaucher Disease - YARGESA is indicated as monotherapy for the treatment of adult patients with mild to moderate type 1 Gaucher disease for whom enzyme replacement therapy is not a ...
-
2 DOSAGE AND ADMINISTRATION2.1 Instructions for Administration - Therapy should be directed by physicians who are knowledgeable in the management of Gaucher disease. The recommended dose for the treatment of adult ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 100 mg of miglustat, white opaque hard gelatin capsules with “709” printed in black on the body.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Peripheral Neuropathy - In clinical trials, cases of peripheral neuropathy have been reported in 3% of Gaucher‘s patients treated with miglustat capsules. All patients receiving miglustat ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: • Peripheral Neuropathy [see Warnings and Precautions (5.1)] • Tremor [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSWhile co-administration of miglustat capsules appeared to increase the clearance of imiglucerase by 70%, these results are not conclusive because of the small number of patients studied and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, miglustat capsules may cause fetal harm when administered to a pregnant woman. Available data from ...
-
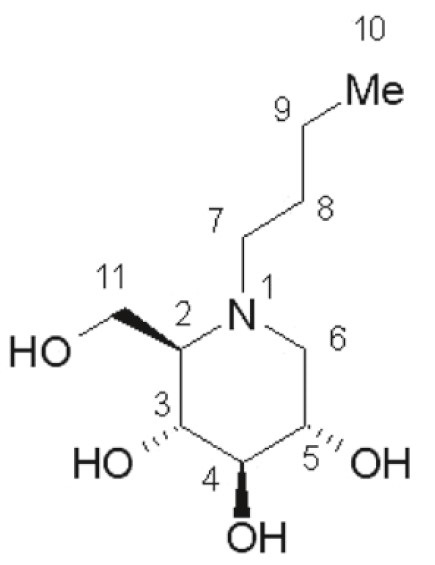
11 DESCRIPTIONYARGESA (miglustat capsules) is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Type 1 Gaucher disease is caused by a functional deficiency of glucocerebrosidase, the enzyme that mediates the degradation of the glycosphingolipid glucosylceramide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Carcinogenesis: Two-year carcinogenicity studies have been conducted with miglustat in CD-1 mice at oral doses up to 500 mg/kg/day ...
-
14 CLINICAL STUDIESThe efficacy of miglustat capsules in type 1 Gaucher disease has been investigated in two open-label, uncontrolled trials and one randomized, open-label, active-controlled trial with enzyme ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGYARGESA is supplied in hard gelatin capsules containing 100 mg miglustat. YARGESA 100 mg capsules are white opaque with “709” printed in black on the body. YARGESA are packed in blister cards ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information) Information for Patients - • Advise patients that the most common serious adverse reactions reported with YARGESA are peripheral neuropathy ...
-
Patient InformationYARGESA (yär ges uh) (miglustat) Capsules - Read this Patient Information before you start taking YARGESA and each time you get a refill. There may be new information. What is YARGESA ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information