Label: DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE OPHTHALMIC SOLUTION solution/ drops
- NDC Code(s): 46708-553-10
- Packager: Alembic Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDorzolamide hydrochloride and timolol maleate ophthalmic solution is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension ...
-
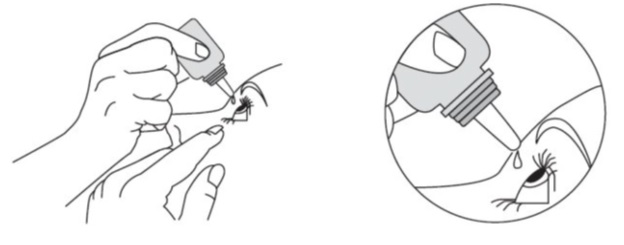
2 DOSAGE AND ADMINISTRATIONThe dose is one drop of dorzolamide hydrochloride and timolol maleate ophthalmic solution in the affected eye(s) two times daily. If more than one topical ophthalmic drug is being used, the drugs ...
-
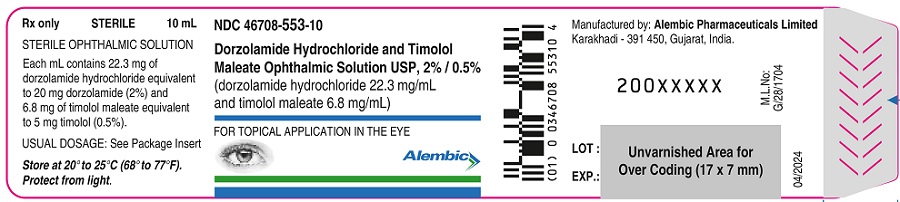
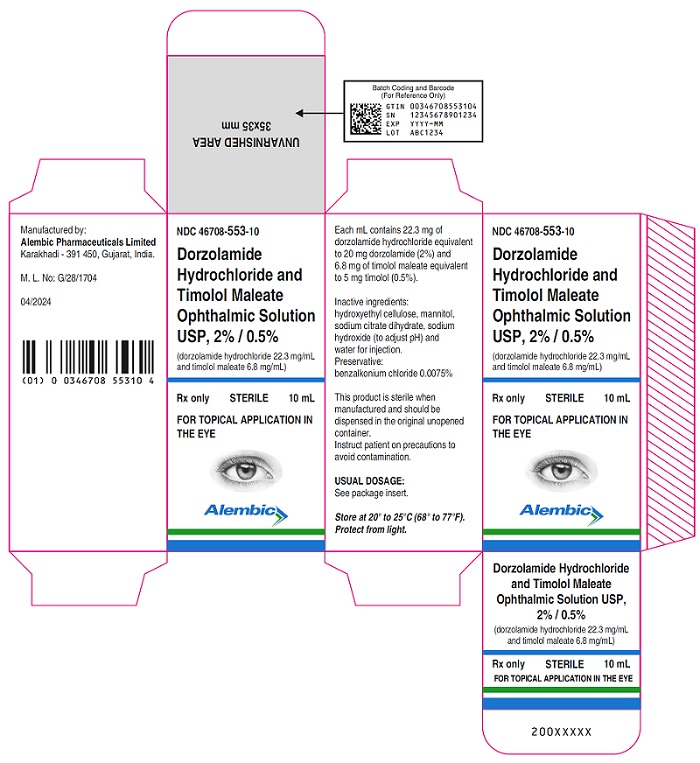
3 DOSAGE FORMS AND STRENGTHSOpthalmic solution containing dorzolamide 20 mg/mL (2%) equivalent to (22.26 mg/mL of dorzolamide hydrochloride) and timolol 5 mg/mL (0.5%)equivalent to (6.83 mg/mL timolol maleate).
-
4 CONTRAINDICATIONS4.1 Asthma, COPD - Dorzolamide hydrochloride and timolol maleate ophthalmic solution is contraindicated in patients with bronchial asthma, a history of bronchial asthma, or severe chronic ...
-
5 WARNINGS AND PRECAUTIONS5.1 Potentiation of Respiratory Reactions Including Asthma - Dorzolamide hydrochloride and timolol maleate ophthalmic solution contains timolol maleate, a beta-adrenergic blocking agent; and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Oral Carbonic Anhydrase Inhibitors - There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects. Developmental toxicity studies with dorzolamide hydrochloride in rabbits at oral doses of ≥2.5 mg/kg/day (37 times the recommended human ophthalmic dose ...
-
10 OVERDOSAGESymptoms consistent with systemic administration of beta-blockers or carbonic anhydrase inhibitors may occur, including electrolyte imbalance, development of an acidotic state, dizziness ...
-
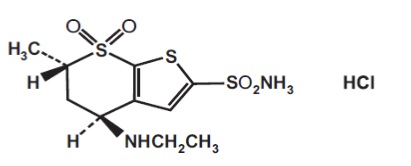
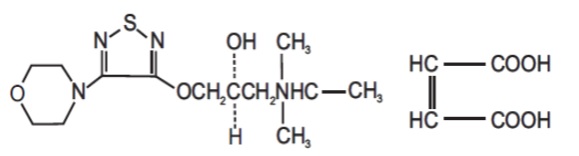
11 DESCRIPTIONDorzolamide hydrochloride and timolol maleate ophthalmic solution is the combination of a topical carbonic anhydrase inhibitor and a topical beta-adrenergic receptor blocking agent. Dorzolamide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dorzolamide hydrochloride and timolol maleate ophthalmic solution is comprised of two components: dorzolamide hydrochloride and timolol maleate. Each of these two ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility - In a two-year study of dorzolamide hydrochloride administered orally to male and female Sprague- Dawley rats, urinary bladder ...
-
14 CLINICAL STUDIESClinical studies of 3 to 15 months duration were conducted to compare the IOP-lowering effect over the course of the day of dorzolamide hydrochloride and timolol maleate ophthalmic solution twice ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDorzolamide hydrochloride and timolol maleate ophthalmic solution 2% / 0.5% is supplied in opaque white low density polyethylene bottle which is closed with white low density polyethylene nozzle ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-Approved patient labeling (Patient Information and Instructions for Use ). Potential for Exacerbation of Asthma and COPD - Dorzolamide hydrochloride and ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELDorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution, USP 2% & 0.5%- Bottle label - Alembic - Dorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution, USP 2% ...
-
INGREDIENTS AND APPEARANCEProduct Information