Label: DOXYCYCLINE HYCLATE tablet, coated
- NDC Code(s): 60505-4382-1, 60505-4382-5, 60505-4382-6, 60505-4384-1, view more
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DOXYCYCLINE HYCLATE TABLETS safely and effectively. See full prescribing information for DOXYCYCLINE HYCLATE TABLETS. DOXYCYCLINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Rickettsial Infections - Doxycycline hyclate tablets are indicated for treatment of Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsial pox, and tick fevers ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - The usual dosage and frequency of administration of doxycycline hyclate tablets differs from that of the other tetracyclines. Exceeding the ...
-
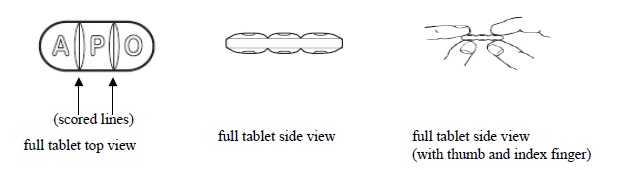
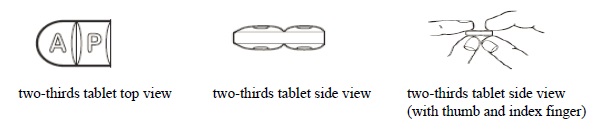
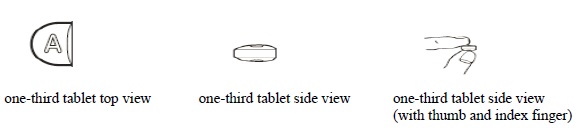
3 DOSAGE FORMS AND STRENGTHSDoxycycline hyclate tablets, USP 75 mg are light teal, round, biconvex film-coated tablets. Engraved "APO" on one side, "D75" on the other side. Doxycycline hyclate tablets, USP 150 mg are mossy ...
-
4 CONTRAINDICATIONSDoxycycline hyclate tablets are contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
5 WARNINGS AND PRECAUTIONS5.1 Tooth Development - The use of doxycycline hyclate tablets during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of ...
-
6 ADVERSE REACTIONSThe following adverse reactions have been identified during clinical trials or post-approval use of tetracycline-class drugs, including doxycycline. Because these reactions are reported ...
-
7 DRUG INTERACTIONS7.1 Anticoagulant Drugs - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Doxycycline hyclate tablets, like other tetracycline-class antibacterial drugs, may cause discoloration deciduous teeth, and reversible inhibition of bone growth ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases ...
-
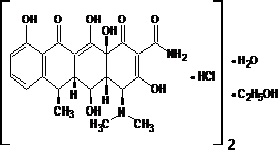
11 DESCRIPTIONDoxycycline hyclate tablets, USP contain doxycycline hyclate, USP a tetracycline class drug synthetically derived from oxytetracycline, in an immediate release formulation for oral ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Doxycycline is a tetracycline-class antimicrobial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - Doxycycline hyclate tablets: Following ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of doxycycline hyclate have not been conducted. However, a 2 year ...
-
15 REFERENCES1 Friedman JM, Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore, MD: The Johns Hopkins University Press: 2000: 149-195. 2 Cziezel AE and Rockenbauer M ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Doxycycline hyclate tablets, USP 75 mg are light teal, round, biconvex film-coated tablets. Engraved "APO" on one side, "D75" on the other side. Each 75 mg tablet contains 86.6 mg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Important Administration and Safety Information for Patients and Caregivers - Advise patients taking ...
-
PATIENT PACKAGE INSERTFDA-Approved Patient Labeling - Instructions for Use - DOXYCYCLINE HYCLATE TABLETS, USP - (dox" i sye’ kleen hye’ klate) for oral use - Read this Instructions for Use before you start using ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTIONRepresentative sample of labeling (see HOW SUPPLIED section for complete listing): PRINCIPAL DISPLAY PANEL - 75 mg BOTTLE LABEL - APOTEX CORP., NDC No. 60505-4382-6 - Doxycycline Hyclate Tablets ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTIONRepresentative sample of labeling (see HOW SUPPLIED section for complete listing): PRINCIPAL DISPLAY PANEL - 150 mg BOTTLE LABEL - APOTEX CORP., NDC No. 60505-4384-6 - Doxycycline Hyclate Tablets ...
-
INGREDIENTS AND APPEARANCEProduct Information