Label: XALKORI- crizotinib capsule
XALKORI- crizotinib capsule, coated pellets
-
NDC Code(s):
0069-0251-60,
0069-0507-60,
0069-1500-60,
0069-8140-20, view more0069-8141-20
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XALKORI® safely and effectively. See full prescribing information for XALKORI.
XALKORI® (crizotinib) capsules, for oral use
XALKORI® (crizotinib) oral pellets
Initial U.S. Approval: 2011RECENT MAJOR CHANGES
Dosage and Administration (2)
9/2023
INDICATIONS AND USAGE
XALKORI is a kinase inhibitor indicated for the treatment of
- •
- adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK) or ROS1-positive as detected by an FDA-approved test. (1.1, 2.1)
- •
- pediatric patients 1 year of age and older and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive. (1.2, 2.3)
- o
- Limitations of Use: The safety and efficacy of XALKORI have not been established in older adults with relapsed or refractory, systemic ALK-positive ALCL.
- •
- adult and pediatric patients 1 year of age and older with unresectable, recurrent, or refractory inflammatory myofibroblastic tumor (IMT) that is ALK-positive. (1.3, 2.3)
DOSAGE AND ADMINISTRATION
- •
- Metastatic NSCLC: The recommended dosage is 250 mg orally twice daily. (2.3)
- •
- Systemic ALCL: The recommended dosage is 280 mg/m2 orally twice daily based on body surface area. (2.3)
- •
- Unresectable IMT:
- •
- See full prescribing information for dosage adjustments by indication for patients with moderate or severe hepatic impairment or severe renal impairment. (2.7, 2.8)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- •
- Hepatotoxicity: Fatal hepatotoxicity has occurred. Monitor with periodic liver testing. Temporarily suspend, dose reduce, or permanently discontinue XALKORI. (2.2, 2.6, 5.1)
- •
- Interstitial Lung Disease (ILD)/Pneumonitis: Permanently discontinue in patients with ILD/pneumonitis. (2.6, 5.2)
- •
- QT Interval Prolongation: Monitor electrocardiograms and electrolytes in patients who have a history of or predisposition for QTc prolongation, or who are taking medications that prolong QT. Temporarily suspend, dose reduce, or permanently discontinue XALKORI. (2.6, 5.3)
- •
- Bradycardia: XALKORI can cause bradycardia. Monitor heart rate and blood pressure regularly. Temporarily suspend, dose reduce, or permanently discontinue XALKORI. (2.6, 5.4)
- •
- Severe Visual Loss: XALKORI can cause visual changes including severe visual loss. Monitor and evaluate for ocular toxicity throughout treatment. Discontinue XALKORI in patients with severe visual loss. (2.2, 2.6, 5.5)
- •
- Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT: XALKORI can cause severe nausea, vomiting, diarrhea, and stomatitis. Provide standard antiemetic and antidiarrheal agents. Temporarily suspend, dose reduce, or permanently discontinue XALKORI. (2.6, 5.6)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.7, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥25%) in adult patients with NSCLC are vision disorders, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, upper respiratory infection, dizziness, and neuropathy. (6.1)
The most common adverse reactions (≥35%) in patients with ALCL are diarrhea, vomiting, nausea, vision disorder, headache, musculoskeletal pain, stomatitis, fatigue, decreased appetite, pyrexia, abdominal pain, cough, and pruritus. Grade 3–4 laboratory abnormalities (≥15%) are neutropenia, lymphopenia, and thrombocytopenia. (6.1)
The most common adverse reactions (≥35%) in adult patients with IMT are vision disorders, nausea, and edema. (6.1)
The most common adverse reactions (≥35%) in pediatric patients with IMT are vomiting, nausea, diarrhea, abdominal pain, rash, vision disorder, upper respiratory tract infection, cough, pyrexia, musculoskeletal pain, fatigue, edema, constipation, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
1.2 Relapsed or Refractory, Systemic ALK-Positive Anaplastic Large Cell Lymphoma
1.3 Unresectable, Recurrent, or Refractory ALK-Positive Inflammatory Myofibroblastic Tumor
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Testing During Treatment with XALKORI
2.3 Recommended Dosage
2.4 Administration
2.5 Concomitant Treatments for Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
2.6 Dosage Modifications for Adverse Reactions
2.7 Dosage Modifications for Moderate and Severe Hepatic Impairment
2.8 Dosage Modification for Severe Renal Impairment
2.9 Dosage Modification for Concomitant Use of Strong CYP3A Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
5.2 Interstitial Lung Disease/Pneumonitis
5.3 QT Interval Prolongation
5.4 Bradycardia
5.5 Severe Visual Loss
5.6 Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XALKORI
7.2 Effect of XALKORI on Other Drugs
7.3 Drugs That Prolong the QT Interval
7.4 Drugs That Cause Bradycardia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
14.2 Relapsed or Refractory, Systemic ALK-Positive Anaplastic Large Cell Lymphoma
14.3 Unresectable, Recurrent, or Refractory ALK-Positive Inflammatory Myofibroblastic Tumor
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
XALKORI is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK) or ROS1-positive as detected by an FDA-approved test [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of metastatic NSCLC with XALKORI based on the presence of ALK or ROS1 positivity in tumor specimens [see Clinical Studies (14.1, 14.2, 14.3)].
Information on FDA-approved tests for the detection of ALK and ROS1 rearrangements in NSCLC is available at http://www.fda.gov/companiondiagnostics.
2.2 Recommended Testing During Treatment with XALKORI
- •
- Monitor liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin, every 2 weeks during the first 2 months of treatment, then once a month, and as clinically indicated, with more frequent repeat testing for increased liver transaminases, alkaline phosphatase, or total bilirubin in patients who develop increased transaminases [see Warnings and Precautions (5.1)].
- •
- Monitor complete blood counts including differential weekly for the first month of therapy and then at least monthly, with more frequent monitoring if Grade 3 or 4 abnormalities, fever, or infection occur [see Adverse Reactions (6.1)].
- •
- For pediatric and young adult patients with ALCL or pediatric patients with IMT, obtain baseline and follow-up ophthalmologic examinations including retinal examination within 1 month of starting XALKORI and every 3 months thereafter [see Warnings and Precautions (5.5)].
2.3 Recommended Dosage
The recommended dosage of XALKORI is provided in Table 1.
Table 1. Recommended Dosage of XALKORI Indication
Recommended Dosage of XALKORI
ALK- or ROS1-Positive Metastatic NSCLC
Adults:
250 mg orally twice dailyRelapsed or Refractory, Systemic ALK-Positive ALCL
Pediatric Patients and Young Adults:
280 mg/m2 orally twice daily*Unresectable, Recurrent, or Refractory ALK-Positive IMT
Adults:
250 mg orally twice dailyPediatric Patients:
280 mg/m2 orally twice daily†Recommended Dosage for Adult Patients with ALK- or ROS1-Positive Metastatic NSCLC
- •
- The recommended dosage for adult patients with ALK- or ROS1-positive metastatic NSCLC is XALKORI capsules 250 mg orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
- •
- For adults who cannot swallow capsules, the recommended dosage of XALKORI pellets is 250 mg (2 x 50 mg + 1 x 150 mg) orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
Recommended Dosage for Pediatric and Young Adult Patients with ALK-Positive ALCL
- •
- The recommended dosage for pediatric patients 1 year of age and older and young adults with relapsed or refractory, systemic ALK-positive ALCL is based on body surface area (BSA) and is provided in Table 2.
- •
- Administer XALKORI capsules or pellets orally, twice daily, with or without food until disease progression or unacceptable toxicity occurs.
Table 2 provides the dosage based on body surface area (BSA) for XALKORI capsules or pellets.
Table 2. Recommended XALKORI Dosage for Pediatric Patients 1 Year of Age and Older and Young Adults With ALK-Positive ALCL Using Either XALKORI Capsules or Pellets - *
- No more than 4 oral pellet shells are to be used for a single dose.
Body Surface Area (BSA)
Recommended XALKORI Dosage to Achieve 280 mg/m2
Twice Daily
Dose Strength Combinations of XALKORI Pellets to Administer*
Dose Strength Combinations of XALKORI Capsules to Administer
0.38 to 0.46 m2
120 mg twice daily
1 x 20 mg + 2 x 50 mg
---
0.47 to 0.51 m2
140 mg twice daily
2 x 20 mg + 2 x 50 mg
---
0.52 to 0.61 m2
150 mg twice daily
1 x 150 mg
---
0.62 to 0.80 m2
200 mg twice daily
1 x 50 mg + 1 x 150 mg
---
0.81 to 0.97 m2
250 mg twice daily
2 x 50 mg + 1 x 150 mg
---
0.98 to 1.16 m2
300 mg twice daily
2 x 150 mg
---
1.17 to 1.33 m2
350 mg twice daily
1 x 50 mg + 2 x 150 mg
---
1.34 to 1.51 m2
400 mg twice daily
2 x 50 mg + 2 x 150 mg
2 x 200 mg
1.52 to 1.69 m2
450 mg twice daily
3 x 150 mg
1 x 200 mg + 1 x 250 mg
1.7 m2 or greater
500 mg twice daily
1 x 50 mg + 3 x 150 mg
2 x 250 mg
Recommended Dosage for Pediatric and Adult Patients with ALK-Positive IMT
- •
- The recommended dosage for adult patients with unresectable, recurrent, or refractory ALK-positive IMT is provided in Table 1.
- •
- The recommended dosage for pediatric patients 1 year of age and older with unresectable, recurrent, or refractory ALK-positive IMT is based on BSA and is provided in Table 3.
- •
- Administer XALKORI capsules or pellets orally twice daily, with or without food, until disease progression or unacceptable toxicity occurs.
Table 3 provides the dosage based on BSA for XALKORI capsules or pellets.
Table 3. Recommended XALKORI Dosage for Pediatric Patients 1 Year of Age and Older with ALK-positive IMT Using Either XALKORI Capsules or Pellets - *
- No more than 4 oral pellet shells are to be used for a single dose.
Body Surface Area (BSA)
Recommended XALKORI Dosage to Achieve 280 mg/m2
Twice Daily
Dose Strength Combinations of XALKORI Pellets to Administer*
Dose Strength Combinations of XALKORI Capsules to Administer
0.38 to 0.46 m2
120 mg twice daily
1 x 20 mg + 2 x 50 mg
---
0.47 to 0.51 m2
140 mg twice daily
2 x 20 mg + 2 x 50 mg
---
0.52 to 0.61 m2
150 mg twice daily
1 x 150 mg
---
0.62 to 0.80 m2
200 mg twice daily
1 x 50 mg + 1 x 150 mg
---
0.81 to 0.97 m2
250 mg twice daily
2 x 50 mg + 1 x 150 mg
---
0.98 to 1.16 m2
300 mg twice daily
2 x 150 mg
---
1.17 to 1.33 m2
350 mg twice daily
1 x 50 mg + 2 x 150 mg
---
1.34 to 1.51 m2
400 mg twice daily
2 x 50 mg + 2 x 150 mg
2 x 200 mg
1.52 to 1.69 m2
450 mg twice daily
3 x 150 mg
1 x 200 mg + 1 x 250 mg
1.7 m2 or greater
500 mg twice daily
1 x 50 mg + 3 x 150 mg
2 x 250 mg
2.4 Administration
- •
- Administer XALKORI capsules or pellets orally, twice daily, with or without food.
- •
- If a dose of XALKORI capsules or pellets is missed, make up that dose unless the next dose is due within 6 hours.
- •
- If vomiting occurs after taking a dose of XALKORI capsules or pellets, do not take an additional dose. Take the next dose at the regular scheduled time.
XALKORI Capsules
- •
- Swallow XALKORI capsules whole, with or without food twice daily.
- •
- Do not chew, crush or split XALKORI capsules.
XALKORI Pellets
- •
- XALKORI pellets are supplied encapsulated in shells.
- •
- Do not chew or crush XALKORI pellets.
- •
- Do not swallow XALKORI pellets encapsulated in the shell.
- •
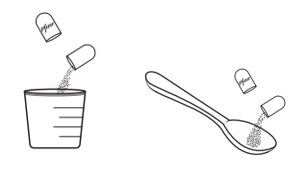
- XALKORI pellets can be administered by 2 options:
- 1.
- Open shell(s) containing XALKORI pellets and empty the contents directly into the patient’s mouth.
- 2.
- Open shell(s) containing XALKORI pellets and empty the contents into a consumer-supplied oral dosing aid (e.g., spoon, medicine cup). Administer XALKORI pellets via the dosing aid directly into the patient’s mouth.
- •
- Immediately after administration, give a sufficient amount of water to ensure that all medication is swallowed.
2.5 Concomitant Treatments for Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
Antiemetics are recommended prior to and during treatment with XALKORI to prevent nausea and vomiting. Provide standard antiemetic and antidiarrheal agents for gastrointestinal toxicities.
Consider intravenous or oral hydration for patients at risk of dehydration, and replace electrolytes as clinically indicated [see Warnings and Precautions (5.6)].
2.6 Dosage Modifications for Adverse Reactions
The recommended dosage modifications for adverse reactions for adult patients with NSCLC or IMT are provided in Table 4.
Table 4. Recommended Dosage Reductions for Adverse Reactions for Adult Patients with NSCLC or IMT Using XALKORI Capsules or Pellets Dose Reduction
Dose and Schedule
First Dose Reduction
200 mg twice daily
Second Dose Reduction
250 mg once daily
Permanently discontinue XALKORI capsules or pellets if unable to tolerate 250 mg taken once daily.
The recommended dosage modifications for adverse reactions for pediatric patients with ALCL or IMT and young adults with ALCL are based on body surface area and are provided in Table 5.
Table 5. Recommended Dosage Reductions for Adverse Reactions for Pediatric Patients with ALCL or IMT and Young Adults with ALCL Using XALKORI Capsules or Pellets - *
- Permanently discontinue in patients who are unable to tolerate XALKORI capsules or pellets after 2 dose reductions.
Body Surface Area
(BSA)
First Dose Reduction
Second Dose Reduction*
Dosage
Dosage Form and Strength to Achieve Recommended Dose Reduction
Dosage
Dosage Form and Strength to Achieve Recommended Dose Reduction
0.38 to 0.46 m2
90 mg
twice daily
Pellets: 2 x 20 mg +
1 x 50 mg
70 mg
twice daily
Pellets: 1 x 20 mg +
1 x 50 mg
0.47 to 0.51 m2
100 mg
twice daily
Pellets: 2 x 50 mg
80 mg
twice daily
Pellets: 4 x 20 mg
0.52 to 0.61 m2
120 mg
twice daily
Pellets: 1 x 20 mg +
2 x 50 mg
90 mg
twice daily
Pellets: 2 x 20 mg +
1 x 50 mg
0.62 to 0.80 m2
150 mg
twice daily
Pellets: 1 x 150 mg
120 mg
twice daily
Pellets: 1 x 20 mg +
2 x 50 mg
0.81 to 0.97 m2
200 mg
twice daily
Pellets: 1 x 50 mg +
1 x 150 mg
150 mg
twice daily
Pellets: 1 x 150 mg
0.98 to 1.16 m2
220 mg
twice daily
Pellets: 1 x 20 mg +
1 x 50 mg +
1 x 150 mg
170 mg
twice daily
Pellets: 1 x 20 mg +
1 x 150 mg
1.17 to 1.33 m2
250 mg
twice daily
Pellets: 2 x 50 mg +
1 x 150 mg
200 mg
twice daily
Pellets: 1 x 50 mg +
1 x 150 mg
1.34 to 1.69 m2
250 mg
twice daily
Pellets: 2 x 50 mg +
1 x 150 mg
Or
Capsule: 1 x 250 mg
200 mg
twice daily
Pellets: 1 x 50 mg +
1 x 150 mg
Or
Capsule: 1 x 200 mg
1.7 m2 or greater
400 mg
twice daily
Pellets: 2 x 50 mg +
2 x 150 mg
Or
Capsule: 2 x 200 mg
250 mg
twice daily
Pellets: 2 x 50 mg +
1 x 150 mg
Or
Capsule: 1 x 250 mg
Recommended Dosage Modifications for Hematologic Adverse Reactions for Adult Patients with NSCLC or IMT
The recommended dosage modifications for hematologic adverse reactions for adult patients with NSCLC or IMT are provided in Table 6.
Table 6. Adult Patients with NSCLC or IMT: XALKORI Dosage Modification – Hematologic Toxicities* Severity of Adverse Reaction†
XALKORI Dosage Modification
Grade 3
Withhold until recovery to Grade 2 or less, then resume at the same dosage.
Grade 4
Withhold until recovery to Grade 2 or less, then resume at next lower dosage.
Monitor complete blood counts including differential weekly for the first month of therapy and then at least monthly, with more frequent monitoring if Grade 3 or 4 abnormalities, fever, or infection occur.
Recommended Dosage Modifications for Hematologic Adverse Reactions in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
The recommended dosage modifications for hematologic adverse reactions in pediatric and young adult patients with ALCL or pediatric patients with IMT are provided in Table 7.
Table 7. Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT: XALKORI Dosage Modification for Hematologic Adverse Reactions - *
- Permanently discontinue in patients who are unable to tolerate XALKORI after 2 dose reductions.
Severity of Adverse Reaction
XALKORI Dosage Modification
Absolute Neutrophil Count (ANC)
Less than 0.5 x 109/L
First occurrence: Withhold until recovery to ANC greater than 1.0 x 109/L, then resume at the next lower dosage.
Second occurrence:- •
- Permanently discontinue for recurrence complicated by febrile neutropenia or infection.
- •
- For uncomplicated Grade 4 neutropenia, either permanently discontinue, or withhold until recovery to ANC greater than 1.0 x 109/L, then resume at the next lower dosage.*
Platelet Count
25 to 50 x 109/L with concurrent bleeding
Withhold until recovery to platelet count greater than 50 x 109/L and bleeding resolves, then resume at the same dosage.
Less than 25 x 109/L
Withhold until recovery to platelet count greater than 50 x 109/L, then resume at the next lower dosage. Permanently discontinue for recurrence.
Anemia
Hemoglobin less than 8 g/dL
Withhold until recovery to hemoglobin 8 g/dL or more, then resume at the same dosage.
Life-threatening anemia; urgent intervention indicated.
Withhold until recovery to hemoglobin 8 g/dL or more, then resume at the next lower dosage. Permanently discontinue for recurrence.
Recommended Dosage Modifications for Non-Hematologic Adverse Reactions
The recommended dosage modifications for non-hematologic adverse reactions are provided in Table 8.
Table 8. All Patients: XALKORI Dosage Modification for Non-Hematologic Adverse Reactions - *
- Grade based on National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
- †
- Adult patients: Heart rate less than 60 beats per minute (bpm); Pediatric patients: Resting heart rate less than the 2.5th percentile per age-specific norms.
- ‡
- Dosage modifications for gastrointestinal toxicity for pediatric patients with ALCL or IMT and young adults with ALCL only.
- §
- Permanently discontinue in patients who are unable to tolerate XALKORI after 2 dose reductions.
Severity of Adverse Reaction*
XALKORI Dosage Modification
Hepatotoxicity [see Warnings and Precautions (5.1)]
Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than 5 times upper limit of normal (ULN) with total bilirubin less than or equal to 1.5 times ULN
Withhold until recovery to baseline or less than or equal to 3 times ULN, then resume at next lower dosage.
ALT or AST greater than 3 times ULN with concurrent total bilirubin greater than 1.5 times ULN (in the absence of cholestasis or hemolysis)
Permanently discontinue.
Interstitial Lung Disease (Pneumonitis) [see Warnings and Precautions (5.2)]
Any grade drug-related interstitial lung disease/pneumonitis
Permanently discontinue.
QT Interval Prolongation [see Warnings and Precautions (5.3)]
QT corrected for heart rate (QTc) greater than 500 ms on at least 2 separate electrocardiograms (ECGs)
Withhold until recovery to baseline or to a QTc less than 481 ms, then resume at next lower dosage.
QTc greater than 500 ms or greater than or equal to 60 ms change from baseline with Torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia
Permanently discontinue.
Bradycardia [see Warnings and Precautions (5.4)]
Bradycardia† (symptomatic, may be severe and medically significant, medical intervention indicated)
Withhold until recovery to a resting heart rate according to the patient’s age (based on the 2.5th percentile per age-specific norms) as follows:
- •
- 1 to less than 2 years: 91 bpm or above
- •
- 2 to 3 years: 82 bpm or above
- •
- 4 to 5 years: 72 bpm or above
- •
- 6 to 8 years: 64 bpm or above
- •
- Older than 8 years: 60 bpm or above
Evaluate concomitant medications known to cause bradycardia, as well as antihypertensive medications.
If contributing concomitant medication is identified and discontinued, or its dose is adjusted, resume at previous dose upon recovery to asymptomatic bradycardia or to the age-specific heart rate provided above.
If no contributing concomitant medication is identified, or if contributing concomitant medications are not discontinued or dose adjusted, resume at reduced dose upon recovery to asymptomatic bradycardia or to the age-specific heart rate provided above.Bradycardia† (life-threatening consequences, urgent intervention indicated)
Permanently discontinue if no contributing concomitant medication is identified.
If contributing concomitant medication is identified and discontinued, or its dose is adjusted, resume at the second dose reduction level in Table 4 or 5 upon recovery to asymptomatic bradycardia or to the heart rate criteria listed for management of symptomatic or severe, medically significant bradycardia, with frequent monitoring.
Permanently discontinue for recurrence.Ocular Toxicity, including Visual Loss [see Warnings and Precautions (5.5)]
Visual Symptoms, Grade 1 (mild symptoms) or Grade 2 (moderate symptoms affecting ability to perform age-appropriate activities of daily living)
Monitor symptoms and report any symptoms to an eye specialist. Consider dose reduction for Grade 2 visual disorders.
Visual Loss (Grade 3 or 4 Ocular Disorder, marked decrease in vision)
Discontinue during evaluation of severe visual loss.
Permanently discontinue XALKORI for Grade 3 or 4 ocular disorders or severe visual loss if no other cause found on evaluation.Gastrointestinal Toxicity‡ [see Warnings and Precautions (5.6)]
Nausea (Grade 3: inadequate oral intake for more than 3 days, medical intervention required)
Grade 3 (despite maximum medical therapy): Withhold until resolved, and then resume at the next lower dose level.§
Vomiting (Grade 3: more than 6 episodes in 24 hours for more than 3 days, medical intervention required, i.e., tube feeding or hospitalization; Grade 4: life-threatening consequences, urgent intervention indicated)
Grade 3 or 4 (despite maximum medical therapy): Withhold until resolved, and then resume at the next lower dose level.§
Diarrhea (Grade 3: increase of 7 or more stools per day over baseline; incontinence; hospitalization indicated; Grade 4: life-threatening consequences, urgent intervention indicated)
Grade 3 or 4 (despite maximum medical therapy): Withhold until resolved, and then resume at the next lower dose level.§
2.7 Dosage Modifications for Moderate and Severe Hepatic Impairment
The recommended dose of XALKORI in patients with moderate hepatic impairment [any aspartate aminotransferase (AST) and total bilirubin greater than 1.5 times the upper limit of normal (ULN) and less than or equal to 3 times ULN] is the first dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
The recommended dose of XALKORI in patients with severe hepatic impairment (any AST and total bilirubin greater than 3 times ULN) is the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL [see Dosage and Administration (2.6), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.8 Dosage Modification for Severe Renal Impairment
The recommended dosage of XALKORI in patients with severe renal impairment [creatinine clearance (CLcr) less than 30 mL/min, calculated using the modified Cockcroft-Gault equation for adult patients and the Schwartz equation for pediatric patients] not requiring dialysis is the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.9 Dosage Modification for Concomitant Use of Strong CYP3A Inhibitors
Avoid concomitant use of strong CYP3A inhibitors [see Drug Interactions (7.1)]. If concomitant use of strong CYP3A inhibitors is unavoidable, reduce the dose of XALKORI to the second dose reduction shown in Table 4 for adult patients with NSCLC or IMT and Table 5 for pediatric patients with ALCL or IMT and young adults with ALCL. After discontinuation of a strong CYP3A inhibitor, resume the XALKORI dose used prior to initiating the strong CYP3A inhibitor.
-
3 DOSAGE FORMS AND STRENGTHS
Capsules:
- •
- 200 mg: hard gelatin capsule, size 1, white opaque body and pink opaque cap, with "Pfizer" on the cap and "CRZ 200" on the body.
- •
- 250 mg: hard gelatin capsule, size 0, pink opaque cap and body, with "Pfizer" on the cap and "CRZ 250" on the body.
Oral Pellets:
- •
- 20 mg: hard gelatin capsule, size 4, white opaque body and light blue opaque cap, printed with black ink “Pfizer” on the cap and “CRZ 20” on the body.
- •
- 50 mg: hard gelatin capsule, size 3, light gray opaque body and gray opaque cap, printed with black ink “Pfizer” on the cap and “CRZ 50” on the body.
- •
- 150 mg: hard gelatin capsule, size 0, light blue opaque body and cap, printed with black ink “Pfizer” on the cap and “CRZ 150” on the body.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
Drug-induced hepatotoxicity with fatal outcome occurred in 0.1% of the 1719 patients treated with XALKORI for NSCLC across clinical trials [see Adverse Reactions (6.1)]. Concurrent elevations in ALT or AST ≥3 times the ULN and total bilirubin ≥2 times the ULN, with normal alkaline phosphatase, occurred in <1% of patients treated with XALKORI. Increased ALT or AST >5 times the ULN occurred in 11% and 6% of patients, respectively. One percent (1.0%) of patients required permanent discontinuation due to elevated transaminases. Increased transaminases generally occurred within the first 2 months of treatment.
In Study ADVL0912, of 121 patients ages 1 to ≤21 years treated with XALKORI for relapsed or refractory tumors including ALCL and IMT, 71% and 79% had increases of AST and ALT, respectively, with increased ALT or AST >5 times the ULN in 6% each. Of the 26 patients with ALCL treated with XALKORI, 65% and 81% had increases of AST and ALT, respectively, with increases >5 times the ULN in 4% each. Of the 14 pediatric patients with IMT treated with XALKORI, 71% had increases of AST and 71% had increases of ALT.
In Study A8081013, of the 7 adult patients with IMT treated with XALKORI, 57% and 43% had increases of AST and ALT, respectively.
Monitor liver function tests, including ALT, AST, and total bilirubin, every 2 weeks during the first 2 months of treatment, then once a month, and as clinically indicated, with more frequent repeat testing for increased liver transaminases, alkaline phosphatase, or total bilirubin in patients who develop increased transaminases. Withhold, reduce dose, or permanently discontinue XALKORI for hepatotoxicity as recommended [see Dosage and Administration (2.6)].
5.2 Interstitial Lung Disease/Pneumonitis
Severe, life-threatening, or fatal interstitial lung disease (ILD)/pneumonitis can occur in patients treated with XALKORI. Across clinical trials in patients with NSCLC (n=1719), 2.9% of XALKORI-treated patients had ILD of any grade, 1% had Grade 3 or 4 ILD, and 0.5% had fatal ILD [see Adverse Reactions (6.1)]. Interstitial lung disease generally occurred within 3 months after the initiation of XALKORI.
In Study ADVL0912, among 121 patients ages 1 to ≤21 years with relapsed or refractory tumors, including ALCL and IMT, ILD occurred in 0.8% of patients.
Monitor patients for pulmonary symptoms indicative of ILD/pneumonitis. Exclude other potential causes of ILD/pneumonitis, and permanently discontinue XALKORI in patients diagnosed with drug-related ILD/pneumonitis [see Dosage and Administration (2.6)].
5.3 QT Interval Prolongation
QTc prolongation can occur in patients treated with XALKORI. Across clinical trials in patients with NSCLC, 2.1% of 1616 patients had QTcF (corrected QT for heart rate by the Fridericia method) greater than or equal to 500 ms and 5% of 1582 patients had an increase from baseline QTcF greater than or equal to 60 ms by automated machine-read evaluation of ECGs.
In Study ADVL0912, QTc prolongation was reported as an adverse reaction in 4.1% of patients, including 8% of patients with ALCL and 7% of pediatric patients with IMT.
Avoid use of XALKORI in patients with congenital long QT syndrome. Monitor ECGs and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or who are taking medications that are known to prolong the QT interval. Withhold, reduce dose, or permanently discontinue XALKORI for QT/QTc interval prolongation as recommended [see Dosage and Administration (2.6), Clinical Pharmacology (12.2)].
5.4 Bradycardia
Symptomatic bradycardia can occur in patients receiving XALKORI. Across clinical trials in patients with NSCLC, bradycardia occurred in 13% of 1719 patients treated with XALKORI. Grade 3 syncope occurred in 2.4% of XALKORI-treated patients and in 0.6% of the chemotherapy-treated patients [see Adverse Reactions (6.1)].
In Study ADVL0912, among 121 patients ages 1 to ≤21 years treated with XALKORI, bradycardia was reported in 14%, including Grade 3 bradycardia in 0.8% of patients. Of the 26 patients with ALCL treated with XALKORI, bradycardia (all Grade 1) was reported in 19%. Of the 14 pediatric patients with IMT treated with XALKORI, bradycardia was reported in 14% of patients, including Grade 3 bradycardia in 7% of patients.
Avoid using XALKORI in combination with other medications known to cause bradycardia (e.g., beta-blockers, nondihydropyridine-calcium channel blockers, clonidine, and digoxin) to the extent possible. Monitor heart rate and blood pressure regularly. If bradycardia occurs, re-evaluate for the use of concomitant medications known to cause bradycardia. Withhold, reduce dose, or permanently discontinue XALKORI for bradycardia as recommended [see Dosage and Administration (2.6)].
5.5 Severe Visual Loss
Across all clinical trials in patients with NSCLC, the incidence of Grade 4 visual field defect with visual loss was 0.2% of 1719 patients [see Adverse Reactions (6.1)]. Optic atrophy and optic nerve disorder have been reported as potential causes of visual loss.
In Study ADVL0912, visual disorders occurred in 46% of 121 patients with XALKORI, including 65% of 26 patients with ALCL and 50% of 14 patients with IMT. Of the 56 patients who experienced visual disorders, one pediatric patient with IMT experienced Grade 3 myopic optic nerve disorder. The most common visual symptoms were blurred vision and visual impairment.
Assessment of visual symptoms for all patients is recommended monthly during treatment. Report new visual symptoms to an eye specialist.
For pediatric and young adult patients with ALCL or pediatric patients with IMT, obtain baseline and follow-up ophthalmologic examinations including retinal examination within 1 month of starting XALKORI and every 3 months thereafter. The ophthalmological evaluation should consist of best corrected visual acuity, retinal photographs, visual fields, optical coherence tomography (OCT), and other evaluations as appropriate [see Dosage and Administration (2.6), Adverse Reactions (6.1)].
Permanently discontinue XALKORI for Grade 3 or 4 ocular disorders or severe visual loss (best corrected vision less than 20/200 in one or both eyes) unless another cause is identified [see Dosage and Administration (2.6)]. There is insufficient information to characterize the risks of resumption of XALKORI in patients who develop visual symptoms or visual loss. A decision to resume XALKORI should consider the potential benefits versus risks to the patient.
5.6 Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
XALKORI can cause severe gastrointestinal toxicities in pediatric and young adult patients with ALCL or pediatric patients with IMT [see Adverse Reactions (6.1)]. In patients with ALCL (n=26), gastrointestinal toxicity occurred in 100% of patients; Grade 3 gastrointestinal toxicity occurred in 27% of patients and included diarrhea, nausea, vomiting, and stomatitis. In pediatric patients with IMT (n=14), vomiting occurred in 93%, nausea occurred in 86%, and diarrhea occurred in 64% of patients.
Provide standard antiemetic and antidiarrheal agents for gastrointestinal toxicities in pediatric and young adult patients with ALCL or pediatric patients with IMT. Antiemetics are recommended prior to and during treatment with XALKORI to prevent nausea and vomiting. If patients develop Grade 3 nausea lasting 3 days or Grade 3 or 4 diarrhea or vomiting despite maximum medical therapy, withhold XALKORI until resolved, and then resume at the next lower dose level. Consider supportive care such as hydration, electrolyte supplementation, and nutritional support as clinically indicated [see Dosage and Administration (2.6)].
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, XALKORI can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of crizotinib in pregnant rats during organogenesis at exposures similar to those observed with the maximum recommended human dose resulted in embryotoxicity and fetotoxicity. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days following the last dose. Advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- •
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.2)]
- •
- QT Interval Prolongation [see Warnings and Precautions (5.3)]
- •
- Bradycardia [see Warnings and Precautions (5.4)]
- •
- Severe Visual Loss [see Warnings and Precautions (5.5)]
- •
- Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data in the Warnings and Precautions reflect exposure to XALKORI in 1719 patients with NSCLC who received XALKORI 250 mg twice daily enrolled on Studies 1 (including an additional 109 patients who crossed over from the control arm), 2, 3, a single-arm trial (n=1063) of ALK-positive NSCLC, and an additional ALK-positive NSCLC expansion cohort of a dose finding study (n=154). The data also reflect exposure to XALKORI in 121 patients ages 1 to ≤21 years with relapsed or refractory tumors, including 26 patients with systemic ALCL and 14 pediatric patients with IMT, in a single-arm trial (Study ADVL0912). The data are also described for 7 adult patients with IMT treated with XALKORI in a single-arm trial (Study A8081013).
ALK- or ROS1-Positive Metastatic NSCLC
The data described below is based primarily on 343 patients with ALK-positive metastatic NSCLC who received XALKORI 250 mg orally twice daily from 2 open-label, randomized, active-controlled trials (Studies 1 and 2). The safety of XALKORI was also evaluated in 50 patients with ROS1-positive metastatic NSCLC from a single-arm study (Study 3).
The most common adverse reactions (≥25%) of XALKORI in patients with NSCLC are vision disorders, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, upper respiratory infection, dizziness, and neuropathy.
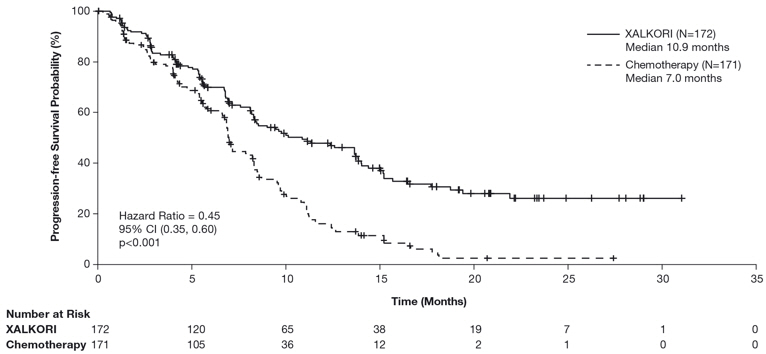
Previously Untreated ALK-Positive Metastatic NSCLC - Study 1 (PROFILE 1014)
The data in Table 9 are derived from 340 patients with ALK-positive metastatic NSCLC who had not received previous systemic treatment for advanced disease who received treatment in a randomized, multicenter, open-label, active-controlled trial (Study 1). Patients in the XALKORI arm (n=171) received XALKORI 250 mg orally twice daily until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. A total of 169 patients in the chemotherapy arm received pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 (n=91) or carboplatin at a dose calculated to produce an AUC of 5 or 6 mg×min/mL (n=78). Chemotherapy was given by intravenous infusion every 3 weeks for up to 6 cycles, in the absence of dose-limiting chemotherapy-related toxicities. After 6 cycles, patients remained on study with no additional anticancer treatment, and tumor assessments continued until documented disease progression.
The median duration of study treatment was 10.9 months for patients in the XALKORI arm and 4.1 months for patients in the chemotherapy arm. Median duration of treatment was 5.2 months for patients who received XALKORI after cross over from chemotherapy. Across the 340 patients who were treated in Study 1, the median age was 53 years; 16% of patients were older than 65 years. A total of 62% of patients were female and 46% were Asian.
Serious adverse events were reported in 34% of patients treated with XALKORI. The most frequent serious adverse events reported in patients treated with XALKORI were dyspnea (4.1%) and pulmonary embolism (2.9%). Fatal adverse events in XALKORI-treated patients occurred in 2.3% patients, consisting of septic shock, acute respiratory failure, and diabetic ketoacidosis.
Dose reductions due to adverse reactions were required in 6% of XALKORI-treated patients. The most frequent adverse reactions that led to dose reduction in these patients were nausea (1.8%) and elevated transaminases (1.8%).
Permanent discontinuation of XALKORI treatment for adverse reactions was 8%. The most frequent adverse reactions that led to permanent discontinuation in XALKORI-treated patients were elevated transaminases (1.2%), hepatotoxicity (1.2%), and ILD (1.2%).
Tables 9 and 10 summarize common adverse reactions and laboratory abnormalities in XALKORI-treated patients.
Table 9. Adverse Reactions Reported at a Higher Incidence (≥5% Higher for All Grades or ≥2% Higher for Grades 3–4) with XALKORI than Chemotherapy in Study 1* Adverse Reaction XALKORI
(N=171)Chemotherapy
(Pemetrexed/Cisplatin or Pemetrexed/Carboplatin)
(N=169)All Grades
(%)Grade 3–4
(%)All Grades
(%)Grade 3–4
(%)- *
- Adverse reactions were graded using NCI CTCAE version 4.0.
Includes cases reported within the clustered terms: - †
- Bradycardia (Bradycardia, Sinus bradycardia).
- ‡
- Vision Disorder (Diplopia, Photophobia, Photopsia, Reduced visual acuity, Blurred vision, Vitreous floaters, Visual impairment).
- §
- Abdominal pain (Abdominal discomfort, Abdominal pain, Lower abdominal pain, Upper abdominal pain, Abdominal tenderness).
- ¶
- Esophagitis (Esophagitis, Esophageal ulcer).
- #
- Edema (Edema, Peripheral edema, Face edema, Generalized edema, Local swelling, Periorbital edema).
- Þ
- Upper respiratory infection (Nasopharyngitis, Pharyngitis, Rhinitis, Upper respiratory tract infection).
- ß
- Dizziness (Balance disorder, Dizziness, Postural dizziness, Presyncope).
Cardiac
Bradycardia†
14
1
1
0
Electrocardiogram QT prolonged
6
2
2
0
Eye
Vision disorder‡
71
1
10
0
Gastrointestinal
Diarrhea
61
2
13
1
Vomiting
46
2
36
3
Constipation
43
2
30
0
Abdominal pain§
26
0
12
0
Dyspepsia
14
0
2
0
Dysphagia
10
1
2
1
Esophagitis¶
6
2
1
0
General
Edema#
49
1
12
1
Pyrexia
19
0
11
1
Infections
Upper respiratory infectionÞ
32
0
12
1
Investigations
Increased weight
8
1
2
0
Musculoskeletal and Connective Tissue
Pain in extremity
16
0
7
0
Muscle spasm
8
0
2
1
Nervous System
Dysgeusia
26
0
5
0
Headache
22
1
15
0
Dizzinessß
18
0
10
1
Additional adverse reactions occurring at an overall incidence between 1% and 60% in patients treated with XALKORI included nausea (56%), decreased appetite (30%), fatigue (29%), neuropathy (21%; gait disturbance, hypoesthesia, muscular weakness, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy, polyneuropathy, sensory disturbance), rash (11%), renal cyst (5%), ILD (1%; ILD, pneumonitis), syncope (1%), and decreased blood testosterone (1%; hypogonadism).
Clinically relevant adverse reactions in <1% of patients who received XALKORI included photosensitivity (0.3%).
Table 10. Laboratory Abnormalities with Grades 3–4 Occurring in ≥4% of XALKORI-Treated Patients in Study 1 XALKORI Chemotherapy Laboratory Abnormality Any Grade
(%)Grade 3–4
(%)Any Grade
(%)Grade 3–4
(%)Additional laboratory test abnormality in patients treated with XALKORI was an increase in creatinine (Any Grade: 99%; Grade 3: 2%; Grade 4: 0%) compared to the chemotherapy arm (Any Grade: 92%; Grade 3: 0%; Grade 4: 1%). Hematology
Neutropenia
52
11
59
16
Lymphopenia
48
7
53
13
Chemistry
Increased ALT
79
15
33
2
Increased AST
66
8
28
1
Hypophosphatemia
32
10
21
6
Previously Treated ALK-Positive Metastatic NSCLC - Study 2 (PROFILE 1007)
The data in Table 11 are derived from 343 patients with ALK-positive metastatic NSCLC enrolled in a randomized, multicenter, active-controlled, open-label trial (Study 2). Patients in the XALKORI arm (n=172) received XALKORI 250 mg orally twice daily until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. A total of 171 patients in the chemotherapy arm received pemetrexed 500 mg/m2 (n=99) or docetaxel 75 mg/m2 (n=72) by intravenous infusion every 3 weeks until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Patients in the chemotherapy arm received pemetrexed unless they had received pemetrexed as part of first-line or maintenance treatment.
The median duration of study treatment was 7.1 months for patients who received XALKORI and 2.8 months for patients who received chemotherapy. Across the 347 patients who were randomized to study treatment (343 received at least 1 dose of study treatment), the median age was 50 years; 14% of patients were older than 65 years. A total of 56% of patients were female and 45% of patients were Asian.
Serious adverse reactions were reported in 37% of patients treated with XALKORI and 23% of patients in the chemotherapy arm. The most frequent serious adverse reactions reported in patients treated with XALKORI were pneumonia (4.1%), pulmonary embolism (3.5%), dyspnea (2.3%), and ILD (2.9%). Fatal adverse reactions in XALKORI-treated patients in Study 2 occurred in 5% of patients, consisting of: acute respiratory distress syndrome, arrhythmia, dyspnea, pneumonia, pneumonitis, pulmonary embolism, ILD, respiratory failure, and sepsis.
Dose reductions due to adverse reactions were required in 16% of XALKORI-treated patients. The most frequent adverse reactions that led to dose reduction in the patients treated with XALKORI were increased ALT (8%) including some patients with concurrent increased AST, QTc prolongation (2.9%), and neutropenia (2.3%).
XALKORI was discontinued for adverse reactions in 15% of patients. The most frequent adverse reactions that led to discontinuation of XALKORI were ILD (1.7%), increased ALT and AST (1.2%), dyspnea (1.2%), and pulmonary embolism (1.2%).
Tables 11 and 12 summarize common adverse reactions and laboratory abnormalities, respectively, in XALKORI-treated patients.
Table 11. Adverse Reactions Reported at a Higher Incidence (≥5% Higher for All Grades or ≥2% Higher for Grades 3–4) with XALKORI than Chemotherapy in Study 2* - *
- Adverse reactions were graded using NCI CTCAE version 4.0.
Includes cases reported within the clustered terms: - †
- Dizziness (Balance disorder, Dizziness, Postural dizziness).
- ‡
- Vision Disorder (Diplopia, Photophobia, Photopsia, Blurred vision, Reduced visual acuity, Visual impairment, Vitreous floaters).
- §
- Bradycardia (Bradycardia, Sinus bradycardia).
- ¶
- Upper respiratory infection (Laryngitis, Nasopharyngitis, Pharyngitis, Rhinitis, Upper respiratory tract infection).
- #
- Pulmonary embolism (Pulmonary artery thrombosis, Pulmonary embolism).
- Þ
- Edema (Face edema, Generalized edema, Local swelling, Localized edema, Edema, Peripheral edema, Periorbital edema).
Adverse Reaction
XALKORI
(N=172)Chemotherapy
(Pemetrexed or Docetaxel)
(N=171)All Grades
(%)Grade 3–4
(%)All Grades
(%)Grade 3–4
(%)Nervous System
Dysgeusia
26
0
9
0
Dizziness†
22
1
8
0
Syncope
3
3
0
0
Eye
Vision disorder‡
60
0
9
0
Cardiac
Electrocardiogram QT prolonged
5
3
0
0
Bradycardia§
5
0
0
0
Investigations
Decreased weight
10
1
4
0
Gastrointestinal
Diarrhea
60
0
19
1
Nausea
55
1
37
1
Vomiting
47
1
18
0
Constipation
42
2
23
0
Dyspepsia
8
0
3
0
Infections
Upper respiratory infection¶
26
0
13
1
Respiratory, Thoracic and Mediastinal
Pulmonary embolism#
6
5
2
2
General
EdemaÞ
31
0
16
0
Additional adverse reactions occurring at an overall incidence between 1% and 30% in patients treated with XALKORI included decreased appetite (27%), fatigue (27%), neuropathy (19%; dysesthesia, gait disturbance, hypoesthesia, muscular weakness, neuralgia, peripheral neuropathy, paresthesia, peripheral sensory neuropathy, polyneuropathy, burning sensation in skin), rash (9%), ILD (4%; acute respiratory distress syndrome, ILD, pneumonitis), renal cyst (4%), esophagitis (2%), hepatic failure (1%), and decreased blood testosterone (1%; hypogonadism).
Clinically relevant adverse reactions in <1% of patients who received XALKORI included photosensitivity (0.4%).
Table 12. Laboratory Abnormalities with Grades 3–4 Occurring in ≥4% of XALKORI-Treated Patients in Study 2 Additional laboratory test abnormality in patients treated with XALKORI was an increase in creatinine (Any Grade: 96%; Grade 3: 1%; Grade 4: 0%) compared to the chemotherapy arm (Any Grade: 72%; Grade 3: 0%; Grade 4: 0%). XALKORI
Chemotherapy
Laboratory Abnormality
Any Grade
(%)Grade 3–4
(%)Any Grade
(%)Grade 3–4
(%)Hematology
Lymphopenia
51
9
60
25
Neutropenia
49
12
28
12
Chemistry
Increased ALT
76
17
38
4
Increased AST
61
9
33
0
Hypophosphatemia
28
5
25
6
Hypokalemia
18
4
10
1
ROS1-Positive Metastatic NSCLC - Study 3 (PROFILE 1001)
The safety profile of XALKORI from Study 3, which was evaluated in 50 patients with ROS1-positive metastatic NSCLC, was generally consistent with the safety profile of XALKORI evaluated in patients with ALK-positive metastatic NSCLC (n=1669). Vision disorders occurred in 92% of patients in Study 3; 90% were Grade 1 and 2% were Grade 2. The median duration of exposure to XALKORI was 34.4 months.
Description of Selected Adverse Reactions in Patients with Metastatic NSCLC
Vision disorders: Vision disorders, most commonly visual impairment, photopsia, blurred vision, or vitreous floaters, occurred in 63% of 1719 patients. The majority (95%) of these patients had Grade 1 visual adverse reactions. There were 0.8% of patients with Grade 3 and 0.2% of patients with Grade 4 visual impairment. Based on the Visual Symptom Assessment Questionnaire (VSAQ-ALK), patients treated with XALKORI in Studies 1 and 2 reported a higher incidence of visual disturbances compared to patients treated with chemotherapy. The onset of vision disorder generally was within the first week of drug administration. The majority of patients on the XALKORI arms in Studies 1 and 2 (>50%) reported visual disturbances which occurred at a frequency of 4–7 days each week, lasted up to 1 minute, and had mild or no impact (scores 0 to 3 out of a maximum score of 10) on daily activities as captured in the VSAQ-ALK questionnaire.
Neuropathy: Neuropathy, most commonly sensory in nature, occurred in 25% of 1719 patients. Most events (95%) were Grade 1 or Grade 2 in severity.
Renal cysts: Renal cysts were experienced by 3.0% of 1719 patients. The majority of renal cysts in XALKORI-treated patients were complex. Local cystic invasion beyond the kidney occurred, in some cases with imaging characteristics suggestive of abscess formation. However, across clinical trials no renal abscesses were confirmed by microbiology tests.
Renal toxicity: The estimated glomerular filtration rate (eGFR) decreased from a baseline median of 96.42 mL/min/1.73 m2 (n=1681) to a median of 80.23 mL/min/1.73 m2 at 2 weeks (n=1499) in patients with ALK-positive advanced NSCLC who received XALKORI in clinical trials. No clinically relevant changes occurred in median eGFR from 12 to 104 weeks of treatment. Median eGFR slightly increased (83.02 mL/min/1.73 m2) 4 weeks after the last dose of XALKORI. Overall, 76% of patients had a decrease in eGFR to <90 mL/min/1.73 m2, 38% had a decrease to eGFR to <60 mL/min/1.73 m2, and 3.6% had a decrease to eGFR to <30 mL/min/1.73 m2.
Relapsed or Refractory, Systemic ALK-Positive ALCL - Study ADVL0912
The safety of XALKORI was evaluated in Study ADVL0912 [see Clinical Studies 14.2], which included 26 patients with relapsed or refractory, systemic ALCL after at least one systemic therapy. Eligible patients were 1 to ≤21 years of age and were required to have an absolute neutrophil count ≥1000/mm3 (750/mm3 if bone marrow was involved), platelet count ≥75,000/mm3 (25,000/mm3 if bone marrow was involved), creatinine clearance ≥70ml/min/1.73m2, and QTc ≤480 msec. The study excluded patients with ALT >2.5 times upper limit of normal (ULN), bilirubin ≤1.5 times ULN, and central nervous system tumors.
Patients with ALCL received XALKORI 165 mg/m2 or 280 mg/m2 orally twice daily until disease progression or unacceptable toxicity. The median duration of exposure was 5.4 months (range 1.8, 82.3 months), with 46% of patients treated for at least 6 months and 35% of patients treated for at least 12 months.
Serious adverse reactions occurred in 35% of patients with ALCL treated with XALKORI. The most frequent serious adverse reactions were neutropenia (12%) and hypotension (8%).
Dose interruptions and dose reductions occurred in 77% and 19% of patients with ALCL, respectively. XALKORI was discontinued for an adverse reaction in 8% of patients.
The most common adverse reactions (≥35%), excluding laboratory abnormalities, were diarrhea, vomiting, nausea, vision disorder, headache, musculoskeletal pain, stomatitis, fatigue, decreased appetite, pyrexia, abdominal pain, cough, and pruritis.
The most common Grade 3 or 4 laboratory abnormalities (≥15%) included neutropenia, lymphopenia, and thrombocytopenia. Grade 4 laboratory abnormalities (≥15%) included neutropenia (62%), lymphopenia (35%), and thrombocytopenia (19%).
Selected adverse reactions are summarized in Table 13.
Table 13. Adverse Reactions in ≥20% of Patients with Systemic ALCL in Study ADVL0912 Adverse reactions were graded using NCI CTCAE version 4.0. - *
- Derived from laboratory values collected in Cycle 1 and adverse reaction data.
- †
- Includes neutrophil count decreased.
- ‡
- Includes lymphocyte count decreased.
- §
- Includes platelet count decreased.
- ¶
- Includes oral pain, oropharyngeal pain, stomatitis.
- #
- Includes blurred vision, visual impairment, photophobia, photopsia, reduced visual acuity, vitreous floaters, cyanopsia, heterophoria, visual field defect.
- Þ
- Includes arthralgia, back pain, myalgia, non-cardiac chest pain, pain in extremity.
- ß
- Includes peripheral edema, face edema, periorbital edema, localized edema.
- à
- Includes rash maculopapular, rash pustular.
- è
- Includes upper respiratory tract infection, pharyngitis, rhinitis, sinusitis.
Adverse Reaction
XALKORI
N=26All Grades
(%)Grade 3–4
(%)Blood and Lymphatic System Disorders*
Neutropenia†
100
77
Lymphopenia‡
58
38
Anemia
54
3.8
Thrombocytopenia§
38
19
Gastrointestinal Disorders
Diarrhea
92
12
Vomiting
92
3.8
Nausea
77
3.8
Abdominal Pain
50
0
Stomatitis¶
46
8
Constipation
31
0
Renal Disorders*
Blood creatinine increased
100
0
Investigations*
ALT increased
81
3.8
AST increased
65
3.8
Hypocalcemia
62
3.8
Hypoalbuminemia
54
0
Hyperglycemia
46
0
Hypomagnesemia
46
0
Hypoglycemia
35
0
Hypokalemia
31
3.8
Hypermagnesemia
27
0
Hyperkalemia
23
0
Nervous System Disorders
Headache
58
3.8
Dysgeusia
23
0
Dizziness
23
0
Eye Disorders
Vision disorders#
65
0
Musculoskeletal Disorders
Musculoskeletal painÞ
58
12
General Disorders
Fatigue
46
0
Pyrexia
38
0
Edemaß
27
0
Chills
23
0
Metabolism and Nutrition Disorders
Decreased appetite
42
0
Skin and Subcutaneous Disorders
Pruritus
35
0
Rashà
23
0
Infections
Upper respiratory tract infectionè
31
0
Respiratory Disorders
Cough
35
0
Rhinitis allergic
23
0
Vascular Disorders
Hypertension
31
0
Clinically relevant adverse reactions in <20% of patients treated with XALKORI included:
- •
- Cardiac disorders: Bradycardia (19%), electrocardiogram QT prolonged (8%)
- •
- Vascular disorders: Hypotension (19%)
- •
- Investigations: Alkaline phosphatase increase (19%), hypernatremia (19%), GGT increase (8%), hyponatremia (12%), hyperuricemia (12%), hypophosphatemia (12%)
- •
- Nervous system disorders: Peripheral neuropathy (12%)
- •
- Gastrointestinal disorders: Esophagitis (8%)
- •
- Blood and lymphatic disorders: Febrile neutropenia (3.8%)
- •
- Musculoskeletal disorders: Muscular weakness (8%)
- •
- Renal disorders: Acute renal injury (8%)
Unresectable, Recurrent, or Refractory ALK-Positive IMT
Study ADVL0912
The safety of XALKORI was evaluated in Study ADVL0912 [see Clinical Studies (14.3)] that included 14 pediatric patients with unresectable, recurrent, or refractory IMT.
Pediatric patients with IMT received XALKORI 280 mg/m2 orally twice daily until disease progression or unacceptable toxicity. Two patients received a lower dose. The median duration of treatment with XALKORI was 20.5 months.
Serious adverse reactions occurred in 7% of pediatric patients with IMT treated with XALKORI. The most frequent serious adverse reaction was neutropenia and hypotension (7% for each).
Dose interruptions due to an adverse reaction occurred in 71% of patients. Dose reductions due to an adverse reaction occurred in 29% of patients. Permanent discontinuation occurred in 29% of patients.
The most common adverse reactions (≥35%) were vomiting, nausea, diarrhea, abdominal pain, rash, vision disorder, upper respiratory tract infection, cough, pyrexia, musculoskeletal pain, fatigue, edema, constipation, and headache.
The most common Grade 3 or 4 laboratory abnormality (>15%) was neutropenia.
Table 14 and Table 15 summarize the adverse reactions and laboratory abnormalities, respectively, in Study ADVL0912.
Table 14. Adverse Reactions in ≥20% of Pediatric Patients with IMT Treated With XALKORI in Study ADVL0912 Adverse reactions were graded using NCI CTCAE version 4.0. - *
- Includes abdominal pain and abdominal pain upper.
- †
- Includes oral pain and oropharyngeal pain.
- ‡
- Includes upper respiratory tract infection, pharyngitis, rhinitis.
- §
- Includes cough and productive cough.
- ¶
- Includes rash maculopapular, rash pustular, dermatitis acneiform.
- #
- Includes face edema, localized edema, periorbital edema, peripheral edema.
- Þ
- Includes pain, bone pain, ear pain.
- ß
- Includes photophobia, photopsia, vision blurred, visual impairment, vitreous floaters.
- à
- Includes arthralgia, myalgia, non-cardiac chest pain, pain in extremity.
Adverse Reaction
XALKORI
N=14All Grades
(%)Grade 3–4
(%)Gastrointestinal Disorders
Vomiting
93
0
Nausea
86
0
Diarrhea
64
7
Abdominal pain*
57
0
Constipation
36
0
Stomatitis†
29
0
Infections
Upper respiratory tract infection‡
64
0
Skin Infection
29
0
Respiratory Disorders
Cough§
64
0
Rhinitis allergic
29
0
Skin and Subcutaneous Disorders
Rash¶
57
0
General Disorders
Pyrexia
50
0
Fatigue
43
0
Edema#
36
7
PainÞ
29
0
Eye Disorders
Vision disordersß
50
0
Musculoskeletal Disorders
Musculoskeletal painà
43
0
Nervous System Disorders
Headache
36
0
Metabolism and Nutrition Disorders
Decreased appetite
29
0
Vascular Disorders
Hypotension
21
7
Clinically relevant adverse reactions in <20% of pediatric patients with IMT treated with XALKORI included:
Cardiac disorders: Bradycardia (14%), electrocardiogram QT prolonged (7%)
Gastrointestinal disorders: Dyspepsia (14%), esophagitis (7%)
Vascular disorders: Hypertension (14%)
Nervous system disorders: Peripheral neuropathy (7%)
Blood and lymphatic disorders: Febrile neutropenia (7%)
Musculoskeletal disorders: Muscular weakness (7%)
Table 15. Laboratory Abnormalities (≥15%) That Worsened from Baseline in Pediatric Patients with IMT Treated With XALKORI in Study ADVL0912 Laboratory Abnormality
Any Grade
(%)Grade 3–4
(%)Chemistry
Increased creatinine
100
0
Decreased calcium
36
0
Increased magnesium
23
0
Decreased phosphate
15
0
Hematology
Decreased neutrophils
64
36
Hepatic
Increased ALT
36
0
Study A8081013
The safety of XALKORI for adult patients with ALK-positive IMT was evaluated in Study A8081013 [see Clinical Studies (14.3)] that included 7 patients with IMT with a median age of 38 years (range 23 to 73). The safety profile of this patient group was generally consistent with the safety profile of XALKORI evaluated in patients with ALK-positive or ROS1-positive NSCLC.
The most frequent adverse reactions (≥20%) were vision disorders, nausea, and edema.
6.2 Postmarketing Experience
The following additional adverse reaction has been identified during post-approval use of XALKORI. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Investigations: Increased blood creatine phosphokinase
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on XALKORI
Strong or Moderate CYP3A Inhibitors
Concomitant use of crizotinib with strong CYP3A inhibitors increases crizotinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions of XALKORI. Avoid concomitant use of strong CYP3A inhibitors. If concomitant use of strong CYP3A inhibitors is unavoidable, reduce the XALKORI dosage [see Dosage and Administration (2.9)]. Avoid grapefruit or grapefruit juice which may also increase plasma concentrations of crizotinib. Use caution with concomitant use of moderate CYP3A inhibitors.
Strong CYP3A Inducers
Concomitant use of crizotinib with strong CYP3A inducers decreases crizotinib plasma concentrations [see Clinical Pharmacology (12.3)], which may decrease the efficacy of XALKORI. Avoid concomitant use of strong CYP3A inducers.
7.2 Effect of XALKORI on Other Drugs
CYP3A Substrates
Concomitant use of crizotinib increases plasma concentrations of CYP3A substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions of these substrates. Avoid concomitant use of XALKORI with CYP3A substrates where minimal concentration changes may lead to serious adverse reactions. If concomitant use of XALKORI is unavoidable, decrease the CYP3A substrate dosage in accordance with approved product labeling.
7.3 Drugs That Prolong the QT Interval
XALKORI can prolong the QT/QTc interval. Avoid concomitant use of XALKORI with drugs that prolong the QT interval [see Warnings and Precautions (5.3), Clinical Pharmacology (12.2)].
7.4 Drugs That Cause Bradycardia
XALKORI can cause bradycardia. Avoid concomitant use of XALKORI with drugs that cause bradycardia (e.g., beta-blockers, non-dihydropyridine calcium channel blockers, clonidine, and digoxin) [see Warnings and Precautions (5.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, XALKORI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of XALKORI in pregnant women. In animal reproduction studies, oral administration of crizotinib to pregnant rats during organogenesis at exposures similar to those expected with the maximum recommended human dose resulted in embryotoxicity and fetotoxicity (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
Crizotinib was administered to pregnant rats and rabbits during organogenesis to study the effects on embryo-fetal development. Postimplantation loss was increased at doses ≥50 mg/kg/day (approximately 0.6 times the recommended human dose based on AUC) in rats. No teratogenic effects were observed in rats at doses up to the maternally toxic dose of 200 mg/kg/day (approximately 2.7 times the recommended human dose based on AUC) or in rabbits at doses of up to 60 mg/kg/day (approximately 1.6 times the recommended human dose based on AUC), though fetal body weights were reduced at these doses.
8.2 Lactation
Risk Summary
There is no information regarding the presence of crizotinib or its metabolites in human milk, or the effects on the breastfed child or on milk production. Because of the potential for adverse reactions in breastfed children, advise women not to breastfeed during treatment with XALKORI and for 45 days after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating XALKORI [see Use in Specific Population (8.1)].
Contraception
XALKORI can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days after the last dose.
Males
Because of the potential for genotoxicity, advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Based on reproductive organ findings in animals, XALKORI may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of XALKORI have been established in pediatric patients 1 year of age and older with relapsed or refractory, systemic ALK-positive ALCL or with unresectable, recurrent, or refractory ALK-positive IMT [see Adverse Reactions (6.1), Clinical Studies (14.2, 14.3)]. The safety and effectiveness have not been established in pediatric patients younger than 1 year of age with ALCL or with IMT, or in any pediatric patients with NSCLC.
In a study that evaluated XALKORI in combination with chemotherapy in pediatric patients with newly diagnosed ALCL (Study ANHL12P1; NCT01979536), 13 of 66 (20%) patients had a Grade 2 or higher thromboembolic event, including pulmonary embolism in 6%. The safety and effectiveness of XALKORI in combination with chemotherapy have not been established in patients with newly diagnosed ALCL.
Juvenile Animal Toxicity Data
Decreased bone formation in growing long bones was observed in immature rats at 150 mg/kg/day following once daily dosing for 28 days (approximately 5.4 times the recommended human dose based on AUC). Other toxicities of potential concern to pediatric patients have not been evaluated in juvenile animals.
8.5 Geriatric Use
Of the total number of patients with ALK-positive metastatic NSCLC in clinical studies of XALKORI (n=1669), 16% were 65 years or older and 3.8% were 75 years or older. No overall differences in safety or effectiveness were observed between these patients and younger patients.
Clinical studies of XALKORI in patients with ROS1-positive metastatic NSCLC did not include sufficient numbers of patients age 65 years and older to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
Crizotinib concentrations increased in patients with pre-existing moderate (any AST and total bilirubin greater than 1.5 times ULN and less than or equal to 3 times ULN) or severe (any AST and total bilirubin greater than 3 times ULN) hepatic impairment [see Clinical Pharmacology (12.3)]. Reduce XALKORI dosage in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.7)]. No dose adjustment is recommended in patients with pre-existing mild hepatic impairment (AST > ULN and total bilirubin less than or equal to 1 times ULN or any AST and total bilirubin greater than 1 times ULN but less than or equal to1.5 times ULN).
8.7 Renal Impairment
Increased exposure to crizotinib occurred in patients with pre-existing severe renal impairment (CLcr less than 30 mL/min calculated using the modified Cockcroft-Gault equation for adult patients and the Schwartz equation for pediatric patients) not requiring dialysis, therefore reduce dosage of XALKORI in these patients [see Dosage and Administration (2.8), Clinical Pharmacology (12.3)]. No dose adjustment is recommended in patients with mild to moderate renal impairment (CLcr 30 to 89 mL/min).
-
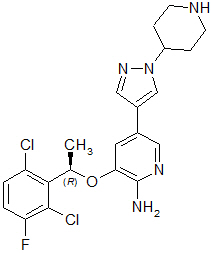
11 DESCRIPTION
Crizotinib is a kinase inhibitor. The molecular formula for crizotinib is C21H22Cl2FN5O and the molecular weight is 450.34 daltons. Crizotinib is described chemically as (R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine.
The chemical structure of crizotinib is shown below:
Crizotinib is a white to pale-yellow powder with a pKa of 9.4 (piperidinium cation) and 5.6 (pyridinium cation). The solubility of crizotinib in aqueous media decreases over the range pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65.
Capsules:
XALKORI (crizotinib) capsules for oral administration are supplied as printed hard-shell capsules containing 250 mg or 200 mg of crizotinib together with colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, and hard gelatin capsule shells as inactive ingredients.
The pink opaque capsule shell components contain gelatin, titanium dioxide, and red iron oxide. The white opaque capsule shell components contain gelatin and titanium dioxide. The printing ink contains shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Oral Pellets:
XALKORI (crizotinib) oral pellets for oral administration are supplied as 20 mg, 50 mg, 150 mg of crizotinib contained in hard gelatin capsules. The inactive ingredients in the uncoated pellets are poloxamer and stearyl alcohol. The film-coating consists of hypromellose, glyceryl monostearate, medium chain triglycerides, polyethylene glycol/macrogol, sucrose, and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), ROS1 (c-ros), and Recepteur d'Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the gene's expression and signaling which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrated concentration-dependent inhibition of ALK, ROS1, and c-Met phosphorylation in cell-based assays using tumor cell lines and demonstrated antitumor activity in mice bearing tumor xenografts that expressed echinoderm microtubule-associated protein-like 4 (EML4)- or nucleophosmin (NPM)-ALK fusion proteins or c-Met.
In vitro, crizotinib induced apoptosis and inhibited proliferation and ALK-mediated signaling in ALCL-derived cell lines (containing NPM-ALK) at clinically achievable exposures. In vivo data obtained in an ALCL-derived mouse model showed complete regression of the tumor at a dose of 100 mg/kg once daily.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In an ECG substudy conducted in 52 patients with ALK-positive NSCLC, the maximum mean QTcF (corrected QT by the Fridericia method) change from baseline was 12.3 ms (2-sided 90% upper CI: 19.5 ms) following administration of XALKORI 250 mg orally twice daily. An exposure-QT analysis suggested a crizotinib plasma concentration-dependent increase in QTcF [see Warnings and Precautions (5.3)].
12.3 Pharmacokinetics
Following XALKORI 250 mg capsules twice daily, steady-state was reached within 15 days with a median accumulation ratio of 4.8. Steady-state minimum concentration (Cmin.ss) and AUC increased in a greater than dose-proportional manner over the dose range of 200 mg to 300 mg twice daily (0.8 to 1.2 times the approved recommended dosage).
Absorption
A single crizotinib capsule dose was absorbed with median time to achieve peak concentration (Tmax) of 4 to 6 hours, and the mean absolute bioavailability of 43% (range: 32% to 66%).
The oral pellets had a comparable crizotinib bioavailability compared with the capsules.
Distribution
The geometric mean volume of distribution (Vss) of crizotinib was 1772 L following a single intravenous dose. Protein binding of crizotinib is 91% and is independent of drug concentration in vitro. Crizotinib is a substrate for P-glycoprotein (P-gp) in vitro. The blood-to-plasma concentration ratio is approximately 1.
Elimination
The mean apparent plasma terminal half-life of crizotinib was 42 hours following single doses of crizotinib in patients. The mean apparent clearance (CL/F) of crizotinib was lower at steady-state (60 L/h) after 250 mg twice daily than after a single 250 mg oral dose (100 L/h).
Specific Populations
No clinically significant difference in crizotinib pharmacokinetics were observed based on age, sex, or ethnicity (Asian, non-Asian). For patients <18 years of age, body weight has a significant effect on the pharmacokinetics of crizotinib, with lower crizotinib exposures observed in patients with higher body weight.
Pediatric Patients
In pediatric patients, crizotinib steady-state exposure increased proportionally with dose over the dose range of 165 mg/m2 to 280 mg/m2 orally twice daily. At a dosing regimen of 280 mg/m2 (approximately 2 times the recommended adult dose), geometric mean (CV%) steady-state maximum plasma concentrations (Cmax) of crizotinib was 621 (73%) ng/mL and AUC0–tau was 6530 (34%) ng∙hr/mL.
Patients with Hepatic Impairment
Steady-state mean crizotinib AUC and Cmax decreased by 9% in patients with mild hepatic impairment (AST >ULN and total bilirubin ≤1 times ULN or any AST and total bilirubin >1 times ULN but ≤1.5 times ULN) compared to patients with normal hepatic function following XALKORI 250 mg orally twice daily.
Steady-state mean crizotinib AUC increased by 14% and Cmax increased by 9% in patients with moderate hepatic impairment (any AST and total bilirubin >1.5 times ULN and ≤3 times ULN) following XALKORI 200 mg orally twice daily compared with patients with normal hepatic function following XALKORI 250 mg orally twice daily.
Mean crizotinib AUC decreased by 35% and Cmax decreased by 27% in patients with severe hepatic impairment (any AST and total bilirubin >3 times ULN) following XALKORI 250 mg orally once daily compared with patients with normal hepatic function following XALKORI 250 mg orally twice daily [see Dosage and Administration (2.7), Use in Specific Populations (8.6)].
Patients with Renal Impairment
Mild or moderate renal impairment (CLcr of 60–89 ml/min or 30–59 ml/min, respectively, calculated using the modified Cockcroft-Gault equation) has no clinically significant effect on the exposure of crizotinib. Following a single 250 mg dose, the mean AUC0–INF of crizotinib increased by 79% and the mean Cmax increased by 34% in patients with severe renal impairment (CLcr <30 mL/min) who did not require dialysis compared to those with normal renal function (CLcr ≥90 mL/min). Similar changes in AUC0–INF and Cmax were observed for the active metabolite of crizotinib [see Dosage and Administration (2.8), Use in Specific Populations (8.7)].
Drug Interaction Studies
Clinical Studies
Gastric Acid Reducing Agents: No clinically significant differences in crizotinib pharmacokinetics were observed when used concomitantly with esomeprazole, a proton pump inhibitor.
Strong CYP3A Inhibitors: Coadministration of a single 150 mg oral dose of crizotinib with ketoconazole, a strong CYP3A inhibitor, increased crizotinib AUC0–INF by 216% and Cmax by 44% compared to crizotinib alone. Coadministration of XALKORI 250 mg orally once daily with itraconazole, a strong CYP3A inhibitor, increased crizotinib steady-state AUC by 57% and Cmax by 33% compared to crizotinib alone [see Drug Interactions (7.1)].
Strong CYP3A Inducers: Coadministration of XALKORI 250 mg orally twice daily with rifampin, a strong CYP3A inducer, decreased crizotinib steady-state AUC0–Tau by 84% and Cmax by 79%, compared to crizotinib alone [see Drug Interactions (7.1)].
CYP3A Substrates: Coadministration of XALKORI 250 mg orally twice daily for 28 days increased AUC0–INF of oral midazolam (CYP3A substrate) 3.7-fold compared to midazolam alone [see Drug Interactions (7.2)].
In Vitro Studies
CYP Enzymes: Crizotinib inhibits CYP2B6 in vitro. Crizotinib does not inhibit CYP1A2, CYP2C8, CYP2C9, CYP2C19, or CYP2D6. Crizotinib does not induce CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP3A.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with crizotinib have not been conducted.
Crizotinib was genotoxic in an in vitro micronucleus assay in Chinese Hamster Ovary cultures, in an in vitro human lymphocyte chromosome aberration assay, and in in vivo rat bone marrow micronucleus assays. Crizotinib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay.
No specific studies with crizotinib have been conducted in animals to evaluate the effect on fertility; however, crizotinib is considered to have the potential to impair reproductive function and fertility in humans based on findings in repeat-dose toxicity studies in the rat. Findings observed in the male reproductive tract included testicular pachytene spermatocyte degeneration in rats given greater than or equal to 50 mg/kg/day for 28 days (greater than 1.7 times the recommended human dose based on AUC). Findings observed in the female reproductive tract included single-cell necrosis of ovarian follicles of a rat given 500 mg/kg/day (approximately 10 times the recommended human dose based on body surface area) for 3 days.
-
14 CLINICAL STUDIES
14.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
Previously Untreated ALK-Positive Metastatic NSCLC - Study 1 (PROFILE 1014; NCT01154140)
The efficacy of XALKORI for the treatment of patients with ALK-positive metastatic NSCLC, who had not received previous systemic treatment for advanced disease, was demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 1). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart fluorescence in situ hybridization (FISH) Probe Kit, prior to randomization. The major efficacy outcome measure was progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as assessed by independent radiology review (IRR) committee. Additional efficacy outcome measures included objective response rate (ORR) as assessed by IRR, DOR, and overall survival (OS). Patient-reported lung cancer symptoms were assessed at baseline and periodically during treatment.
Patients were randomized to receive XALKORI (n=172) or chemotherapy (n=171). Randomization was stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0–1, 2), race (Asian, non-Asian), and brain metastases (present, absent). Patients in the XALKORI arm received XALKORI 250 mg orally twice daily until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Chemotherapy consisted of pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 or carboplatin AUC of 5 or 6 mg×min/mL by intravenous infusion every 3 weeks for up to 6 cycles. Patients in the chemotherapy arm were not permitted to receive maintenance chemotherapy. At the time of documented disease progression, as per independent radiology review, patients randomized to chemotherapy were offered XALKORI.
The demographic characteristics of the overall study population were 62% female, median age of 53 years, baseline ECOG performance status 0 or 1 (95%), 51% White and 46% Asian, 4% current smokers, 32% past smokers, and 64% never smokers. The disease characteristics of the overall study population were metastatic disease in 98% of patients, 92% of patients' tumors were classified as adenocarcinoma histology, 27% of patients had brain metastases, and 7% received systemic chemotherapy as adjuvant or neoadjuvant therapy. At the time of the final analysis of overall survival, 84% of patients randomized to the chemotherapy arm subsequently received XALKORI.
Study 1 demonstrated a statistically significant improvement in PFS in patients treated with XALKORI. There was no statistically significant difference in OS between patients treated with XALKORI and patients treated with chemotherapy. Table 16 and Figure 1 summarize the efficacy results. Exploratory patient-reported symptom measures of baseline and post-treatment dyspnea, cough, and chest pain suggested a delay in time to development of or worsening of dyspnea, but not cough or chest pain, in patients treated with XALKORI as compared to chemotherapy. The patient-reported delay in onset or worsening of dyspnea may be an overestimation because patients were not blinded to treatment assignment.
Table 16. Previously Untreated ALK-Positive Metastatic NSCLC - Efficacy Results in Study 1 XALKORI
(N=172)Chemotherapy
(N=171)HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; NR=not reached; CR=complete response; PR=partial response. Progression-Free Survival (Based on IRR)
Number of Events (%)
100 (58%)
137 (80%)
Progressive Disease
89 (52%)
132 (77%)
Death
11 (6%)
5 (3%)
Median, Months (95% CI)
10.9 (8.3, 13.9)
7.0 (6.8, 8.2)
HR (95% CI)*
0.45 (0.35, 0.60)
p-value†
<0.001
Overall Survival
Number of Events (%)
71 (41%)
81 (47%)
Median, Months (95% CI)
NR (45.8, NR)
47.5 (32.2, NR)
HR (95% CI)*
0.76 (0.55, 1.05)
p-value†
0.098
Tumor Responses (Based on IRR)
Objective Response Rate % (95% CI)
74% (67, 81)
45% (37, 53)
CR, n (%)
3 (1.7%)
2 (1.2%)
PR, n (%)
125 (73%)
75 (44%)
p-value‡
<0.001
Duration of Response
Median, Months (95% CI)
11.3 (8.1, 13.8)
5.3 (4.1, 5.8)
Previously Treated ALK-Positive Metastatic NSCLC - Study 2 (PROFILE 1007; NCT00932893)
The efficacy of XALKORI as monotherapy for the treatment of 347 patients with ALK-positive metastatic NSCLC, previously treated with 1 platinum-based chemotherapy regimen, were demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 2). The major efficacy outcome was PFS according to RECIST version 1.1 as assessed by IRR. Additional efficacy outcomes included ORR as assessed by IRR, DOR, and OS.
Patients were randomized to receive XALKORI 250 mg orally twice daily (n=173) or chemotherapy (n=174). Chemotherapy consisted of pemetrexed 500 mg/m2 (if pemetrexed-naïve; n=99) or docetaxel 75 mg/m2 (n=72) intravenously (IV) every 21 days. Patients in both treatment arms continued treatment until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Randomization was stratified by ECOG performance status (0–1, 2), brain metastases (present, absent), and prior EGFR tyrosine kinase inhibitor treatment (yes, no). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart FISH Probe Kit, prior to randomization.
The demographic characteristics of the overall study population were 56% female, median age of 50 years, baseline ECOG performance status 0 or 1 (90%), 52% White and 45% Asian, 4% current smokers, 33% past smokers, and 63% never smokers. The disease characteristics of the overall study population were metastatic disease in at least 95% of patients and at least 93% of patients' tumors were classified as adenocarcinoma histology. At the time of the final analysis of overall survival, 89% of patients randomized to the chemotherapy arm subsequently received XALKORI.
Study 2 demonstrated a statistically significant improvement in PFS in the patients treated with XALKORI. Table 17 and Figure 2 summarize the efficacy results.
Table 17. Previously Treated ALK-Positive Metastatic NSCLC - Efficacy Results in Study 2 XALKORI
(N=173)Chemotherapy
(N=174)HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; CR=complete response; PR=partial response. Progression-Free Survival (Based on IRR)
Number of Events (%)
100 (58%)
127 (73%)
Progressive Disease
84 (49%)
119 (68%)
Death
16 (9%)
8 (5%)
Median, Months (95% CI)
7.7 (6.0, 8.8)
3.0* (2.6, 4.3)
HR (95% CI)†
0.49 (0.37, 0.64)
p-value‡
<0.001
Overall Survival
Number of Events (%)
116 (67%)
126 (72%)
Median, Months (95% CI)
21.7 (18.9,30.5)
21.9 (16.8,26.0)
HR (95% CI)†
0.85 (0.66, 1.10)
p-value‡
0.229
Tumor Responses (Based on IRR)
Objective Response Rate % (95% CI)
65% (58, 72)
20% (14, 26)
CR, n (%)
1 (0.6%)
0
PR, n (%)
112 (65%)
34 (20%)
p-value§
<0.001
Duration of Response
Median, Months (95% CI)
7.4 (6.1, 9.7)
5.6 (3.4, 8.3)
ROS1-Positive Metastatic NSCLC - Study 3 (PROFILE 1001; NCT00585195)
The efficacy and safety of XALKORI was investigated in a multicenter, single-arm study (Study 3), in which patients with ROS1-positive metastatic NSCLC received XALKORI 250 mg orally twice daily. Patients were required to have histologically-confirmed advanced NSCLC with a ROS1 rearrangement, age 18 years or older, ECOG performance status of 0, 1, or 2, and measurable disease. The efficacy outcome measures were ORR and DOR according to RECIST version 1.0 as assessed by IRR and investigator, with imaging performed every 8 weeks for the first 60 weeks.
Baseline demographic and disease characteristics were female (56%), median age of 53 years, baseline ECOG performance status of 0 or 1 (98%), White (54%), Asian (42%), past smokers (22%), never smokers (78%), metastatic disease (92%), adenocarcinoma (96%), no prior systemic therapy for metastatic disease (14%), and prior platinum-based chemotherapy for metastatic disease (80%). The ROS1 status of NSCLC tissue samples was determined by laboratory-developed break-apart FISH (96%) or RT-PCR (4%) clinical trial assays. For assessment by FISH, ROS1 positivity required that ≥15% of a minimum of 50 evaluated nuclei contained a ROS1 gene rearrangement.
Efficacy results are summarized in Table 18.
Table 18. ROS1-Positive Metastatic NSCLC - Results* in Study 3 Efficacy Parameters IRR
(N=50)Investigator-Assessed
(N=50)IRR=independent radiology review; CI=confidence interval; NR=not reached. - *
- As assessed by RECIST version 1.0.
Objective Response Rate (95% CI)
66% (51, 79)
72% (58, 84)
Complete Response, n
1
5
Partial Response, n
32
31
Duration of Response
Median, Months (95% CI)
18.3 (12.7, NR)
NR (14.5, NR)
14.2 Relapsed or Refractory, Systemic ALK-Positive Anaplastic Large Cell Lymphoma
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single arm, open-label study in patients 1 to ≤21 years of age that included 26 patients with relapsed or refractory, systemic ALK-positive ALCL after at least one systemic treatment. ALK-positive status (confirmation of an ALK fusion) was determined locally by immunohistochemistry or fluorescence in situ hybridization. The study excluded patients with primary cutaneous ALCL or central nervous system involvement by lymphoma.
Patients received XALKORI 280 mg/m2 (20 patients) or 165 mg/m2 (6 patients) orally twice daily until disease progression or unacceptable toxicity. Patients were permitted to discontinue XALKORI to undergo hematopoietic stem cell transplantation (HSCT).
Of the 26 patients evaluated, the median age was 11 years (range: 3 to 20); 69% were male, 54% were White, 19% Black, 8% Asian. Patient enrollment by age category was 4 patients from 3 to <6 years, 11 patients from 6 to <12 years, 7 patients from 12 to <18 years, and 4 patients from 18 to ≤21 years.
All patients had received multi-agent systemic therapy, 2 (8%) had received a prior HSCT and 4 (15%) had received at least 3 prior therapies.
Efficacy was based on objective response rate and duration of response, as assessed by an independent review committee (Table 19). The median time to first response was 3.9 weeks (range: 3.5 to 9.1 weeks).
Table 19. Efficacy in Relapsed or Refractory, Systemic ALK-Positive ALCL Efficacy Parameter N=26 CI=confidence interval; N/n=number of patients 88% (71, 96)
Complete response, n
21 (81%)
Partial response, n
2 (8%)
Duration of response‡
Patients maintaining response at 3 months, n/N
13/23 (57%)
Patients maintaining response at 6 months, n/N
9/23 (39%)
Patients maintaining response at 12 months, n/N
5/23 (22%)
14.3 Unresectable, Recurrent, or Refractory ALK-Positive Inflammatory Myofibroblastic Tumor
Pediatric Patients with ALK-positive IMT
Study ADVL0912
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single-arm, open-label study in patients 1 to ≤21 years of age that included 14 pediatric patients with unresectable, recurrent, or refractory ALK-positive IMT. Patients were required to have an ALK fusion determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients (n=12) received XALKORI 280 mg/m2 twice daily until disease progression or unacceptable toxicity. Two patients received a lower dose.
The demographic characteristics were median age 6.5 years (range: 2 to 13); 64% female; 71% White; 7% Black, 21% unknown; 21% Hispanic; and 71% had a Lansky/Karnofsky Score of 100. Patient enrollment by age was 4 patients from 2 to <6 years, 8 patients from 6 to <12 years, and 2 patients from 12 to <18 years.
A total of 12 (86%) patients received prior therapy. The most common prior therapy was surgery (57%).
The major efficacy outcome was objective response rate according to RECIST version 1.0 as assessed by an independent review committee (Table 20).
Table 20. Efficacy in Pediatric Patients with Unresectable, Recurrent, or Relapsed ALK-Positive IMT Efficacy Parameter N=14 CI=confidence interval; N/n=number of patients. Objective response rate (95% CI, %)*
86% (57, 98)
Complete response, n (%)
5 (36)
Partial response n (%)
7 (50)
Duration of response†
N=12
≥6 months, n (%)
7 (58)
≥12 months, n (%)
7 (58)
Adult Patients with ALK-positive IMT
Study A8081013
The efficacy of XALKORI was evaluated in Study A8081013 (NCT01121588), a multicenter, single-arm, open-label study that included 7 adult patients with unresectable, recurrent, or refractory ALK-positive IMT. ALK fusion was determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients received XALKORI 250 mg twice daily.
The demographic characteristics were median age 38 years (range: 23 to 73); 57% male; 57% White, 43% Asian; and 86% ECOG performance status of 0 or 1. Two (29%) patients had at least one prior systemic treatment.
The major efficacy outcome was objective response rate according to RECIST version 1.1 per investigator assessment. For the 7 patients with ALK-positive IMT, 5 experienced a response including 1 complete response. The DOR was ≥6 months for all 5 patients and ≥12 months for 2 patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Capsules:
- •
- 200 mg capsules
Hard gelatin capsule with pink opaque cap and white opaque body, printed with black ink "Pfizer" on the cap, "CRZ 200" on the body; available in:
Bottles of 60 capsules: NDC 0069-8141-20 - •
- 250 mg capsules
Hard gelatin capsule with pink opaque cap and body, printed with black ink "Pfizer" on the cap, "CRZ 250" on the body; available in:
Bottles of 60 capsules: NDC 0069-8140-20
Store at room temperature 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Oral Pellets:
- •
- 20 mg oral pellets
Hard gelatin capsule, size 4, light blue opaque cap and white opaque body, printed with black ink “Pfizer” on the cap, “CRZ 20” on the body; available in:
Bottles of 60 capsules: NDC 0069-0251-60 - •
- 50 mg oral pellets
Hard gelatin capsule, size 3, gray opaque cap and light gray opaque body, printed with black ink “Pfizer” on the cap, “CRZ 50” on the body; available in:
Bottles of 60 capsules: NDC 0069-0507-60 - •
- 150 mg oral pellets
Hard gelatin capsule, size 0, light blue opaque cap and body, printed with black ink “Pfizer” on the cap, “CRZ 150” on the body; available in:
Bottles of 60 capsules: NDC 0069-1500-60
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide; Instructions for Use).
Hepatotoxicity
Inform patients to immediately report symptoms of hepatotoxicity [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (Pneumonitis)
Advise patients to immediately report any new or worsening pulmonary symptoms [see Warnings and Precautions (5.2)].
Bradycardia
Advise patients to report any symptoms of bradycardia and to inform their healthcare provider about the use of any heart or blood pressure medications [see Warnings and Precautions (5.4)].
Severe Visual Loss
Inform patients of the potential risk of severe visual loss and to immediately contact their healthcare provider if they develop severe visual loss. Inform patients that visual changes such as perceived flashes of light, blurry vision, light sensitivity, and floaters are commonly reported adverse reactions and may occur while driving or operating machinery. The onset of visual disorders most commonly occurs during the first week of treatment [see Warnings and Precautions (5.5), Adverse Reactions (6)].
Gastrointestinal Toxicity in Pediatric and Young Adult Patients with ALCL or Pediatric Patients with IMT
Inform patients with ALCL or pediatric patients with IMT of the risk of severe nausea, vomiting, diarrhea, and stomatitis. Advise patients to immediately inform their healthcare provider of problems with swallowing, vomiting, or diarrhea [see Warnings and Precautions (5.6)].
Drug Interactions
Inform patients to avoid grapefruit or grapefruit juice while taking XALKORI. Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)].
Photosensitivity
Inform patients of the signs and symptoms of photosensitivity. Advise patients to avoid prolonged sun exposure and to use sunscreen or protective clothing during treatment with XALKORI [see Adverse Reactions (6.1)].
Dosage and Administration
Advise patients to take XALKORI with or without food. If a patient misses a dose, advise the patient to take it as soon as remembered unless it is less than 6 hours until the next dose, in which case, advise the patient not to take the missed dose. If a patient vomits after taking a dose of XALKORI, advise the patient not to take an extra dose, but to take the next dose at the regular time [see Dosage and Administration (2.4)].
Capsules:
Advise patients to swallow XALKORI capsules whole [see Dosage and Administration (2.4)].
Oral Pellets:
Inform patient or caregiver to open the encapsulated XALKORI oral pellets and administer the oral pellets directly in the patient’s mouth or with a consumer-supplied oral dosing aid, for example a spoon or medicine cup. Advise patient or caregiver that the oral pellets are not to be chewed and to give a sufficient amount of water after pellets are administered to ensure all oral pellets are swallowed [see Dosage and Administration (2.4)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential to use effective contraception during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use condoms during treatment with XALKORI and for 90 days after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise females not to breastfeed during treatment with XALKORI and for 45 days after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential of the potential for reduced fertility from XALKORI [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
-
SPL UNCLASSIFIED SECTION
This product's labeling may have been updated. For the most recent prescribing information, please visit www.Pfizer.com. For medical information about XALKORI, please visit www.pfizermedinfo.com or call 1-800-438-1985.
LAB-0440-28.0
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: Sep 2023 MEDICATION GUIDE
XALKORI® (zal-KOR-ee)
(crizotinib)
capsulesXALKORI® (zal-KOR-ee)
(crizotinib)
oral pelletsWhat is the most important information I should know about XALKORI?
XALKORI may cause serious side effects, including:- •
- Liver problems. XALKORI may cause life-threatening liver injury that may lead to death. Your healthcare provider should do blood tests to check your liver every 2 weeks during the first 2 months of treatment with XALKORI, then 1 time a month and as recommended by your healthcare provider during treatment. Tell your healthcare provider right away if you develop any of the following new or worsening symptoms:
- o
- yellowing of your skin or the white part of your eyes
- o
- severe tiredness
- o
- dark or brown (tea color) urine
- o
- nausea or vomiting
- o
- decreased appetite
- o
- pain on the right side of your stomach
- o
- bleed or bruise more easily than normal
- o
- itching
- •
-
Lung problems (pneumonitis). XALKORI may cause life-threatening lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you have any new or worsening symptoms, including:
- o
- trouble breathing or shortness of breath
- o
- cough with or without mucous
- o
- fever
- •
- Heart problems. XALKORI may cause very slow, very fast, or abnormal heartbeats. Your healthcare provider may check your pulse rate and blood pressure regularly during treatment with XALKORI. Tell your healthcare provider right away if you feel dizzy or faint or have abnormal heartbeats. Tell your healthcare provider if you take any heart or blood pressure medicines.
- •
- Severe vision problems. Vision problems are common with XALKORI. These problems usually happen within 1 week of starting treatment with XALKORI. Vision problems with XALKORI can be severe and may cause partial or complete loss of vision in one or both eyes. Your healthcare provider may hold or permanently stop your treatment with XALKORI and refer you to an eye specialist if any vision problems develop during treatment with XALKORI. Tell your healthcare provider right away if you have any new vision problems, loss of vision or any change in vision, including:
- o
- double vision
- o
- seeing flashes of light
- o
- blurry vision
- o
- light hurting your eyes
- o
- new or increased floaters
In addition, for children or young adults taking XALKORI to treat anaplastic large cell lymphoma (ALCL) or children taking XALKORI to treat inflammatory myofibroblastic tumor (IMT):
Your healthcare provider may refer you to an eye specialist before starting XALKORI, and within 1 month of starting XALKORI to check for vision problems. You should have an eye examination every 3 months during treatment with XALKORI and more often if there are any new vision problems.- •
-
Severe stomach, intestine, and mouth (gastrointestinal) problems in children or young adults with ALCL or children with IMT. XALKORI may cause severe diarrhea, nausea, vomiting, or mouth sores. Tell your healthcare provider right away if problems with swallowing, vomiting, or diarrhea develop during treatment with XALKORI.
- o
- Your healthcare provider may give medicines as needed to prevent or treat diarrhea, nausea, and vomiting.
- o
- Your healthcare provider may recommend drinking more fluids or may prescribe electrolyte supplements or other kinds of nutritional support if severe symptoms develop.
See "What are possible side effects of XALKORI?" for more information about side effects.
What is XALKORI?
XALKORI is a prescription medicine that is used to treat:- •
- adults with non-small cell lung cancer (NSCLC) that has spread to other parts of the body and is caused by a defect in either a gene called ALK (anaplastic lymphoma kinase) or a gene called ROS1.
- •
- children 1 year of age and older and young adults with ALCL when your ALCL with a defect in a gene called ALK has returned or you have tried a treatment and it did not work or is no longer working.
- o
- It is not known if XALKORI is safe and effective in older adults with ALCL.
- •
- adults and children 1 year of age and older with IMT when your IMT with a defect in a gene called ALK cannot be removed by surgery, has returned, or you have tried a treatment and it did not work or is no longer working.
It is not known if XALKORI is safe and effective in children younger than 1 year of age with ALCL or IMT, or in any children with NSCLC.
Before taking XALKORI, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have liver or kidney problems
- •
- have lung problems
- •
- have heart problems, including a condition called long QT syndrome
- •
- have vision or eye problems
- •
- are pregnant or plan to become pregnant. XALKORI can harm your unborn baby.
Females who are able to become pregnant:- o
- Your healthcare provider will check to see if you are pregnant before starting treatment with XALKORI.
- o
- Effective birth control (contraception) should be used during treatment with XALKORI and for 45 days after the last dose of XALKORI.
- o
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with XALKORI.
-
Males who have female partners who can become pregnant:
- o
- You should use condoms during treatment with XALKORI and for 90 days after the last dose of XALKORI.
- •
- are breastfeeding or plan to breastfeed. It is not known if XALKORI passes into your breast milk. Do not breastfeed during treatment with XALKORI and for 45 days after the last dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XALKORI can affect the way other medicines work, and other medicines can affect how XALKORI works.
How should I take XALKORI?
- •
- Take XALKORI exactly as prescribed by your healthcare provider.
XALKORI comes as capsules and oral pellets.
- •
-
If your healthcare provider prescribes XALKORI capsules:
- o
- Swallow XALKORI capsules whole. Do not chew, crush or split XALKORI capsules.
- o
- Take XALKORI capsules with or without food.
- •
-
If your healthcare provider prescribes XALKORI oral pellets:
- o
- See the Instructions for Use at the end of this Medication Guide for instructions about the right way to prepare and give or take XALKORI oral pellets.
- o
- XALKORI oral pellets should be poured from the shell(s) directly into the mouth, or given or taken with a spoon or medicine cup. Right after giving or taking the oral pellets, give or drink enough water to make sure all oral pellets are swallowed.
- o
- Do not swallow shells containing XALKORI oral pellets.
- o
- Do not chew or crush the XALKORI oral pellets.
- o
- Take XALKORI oral pellets with or without food.
- •
- Your healthcare provider will check your blood cell counts weekly during the first month of treatment with XALKORI and then at least monthly during treatment.
- •
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with XALKORI if you have certain side effects. Do not change the dose or stop treatment with XALKORI unless your healthcare provider tells you to.
- •
- If a dose of XALKORI is missed, it should be taken as soon as you remember. If it is close to the time of the next dose (within 6 hours), the missed dose should be skipped, and the next dose should be taken at the regular time.
- •
- If you vomit after taking a dose of XALKORI, do not take an extra dose. The next dose should be taken at the regular time.
- •
- XALKORI should be given to children under adult supervision.
What should I avoid while taking XALKORI?
- •
- Do not drink grapefruit juice, eat grapefruit or take supplements containing grapefruit extract during treatment with XALKORI. These may increase the amount of XALKORI in the blood.
- •
- XALKORI can cause changes in vision, dizziness, and tiredness. Do not drive or operate machinery if you have any of these symptoms.
- •
- Avoid spending prolonged time in sunlight. XALKORI can make your skin sensitive to the sun (photosensitivity), and you may burn more easily. You should use sunscreen and wear protective clothing that covers your skin to help protect against sunburn if you have to be in the sunlight during treatment with XALKORI.
What are the possible side effects of XALKORI?
XALKORI may cause serious side effects, including:The most common side effects of XALKORI in adults with NSCLC include:
- •
- vision problems
- •
- nausea, diarrhea, or vomiting
- •
- swelling of your hands, feet, face, or eyes
- •
- constipation
- •
- increased liver function blood tests
- •
- tiredness
- •
- decreased appetite
- •
- upper respiratory infection
- •
- dizziness
- •
- feeling of numbness or tingling in your arms or legs
The most common side effects of XALKORI in people with ALCL include:
- •
- diarrhea, vomiting, or nausea
- •
- vision problems
- •
- headache
- •
- muscle and joint pain
- •
- mouth sores
- •
- tiredness
- •
- decreased appetite
- •
- fever
- •
- stomach-area (abdominal) pain
- •
- cough
- •
- itchy skin
- •
- low blood counts
The most common side effects of XALKORI in adults with IMT include:
- •
- vision problems
- •
- nausea
- •
- swelling of your hands, feet, face, or eyes
The most common side effects of XALKORI in children with IMT include:
- •
- vomiting, nausea, or diarrhea
- •
- stomach-area (abdominal) pain
- •
- rash
- •
- vision problems
- •
- upper respiratory infection
- •
- cough
- •
- fever
- •
- muscle and joint pain
- •
- tiredness
- •
- swelling of your hands, feet, face, or eyes
- •
- constipation
- •
- headache
XALKORI may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of XALKORI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store XALKORI?
- •
- Store XALKORI capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep XALKORI and all medicines out of the reach of children.
General information about the safe and effective use of XALKORI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XALKORI for a condition for which it was not prescribed. Do not give XALKORI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about XALKORI that is written for health professionals.What are the ingredients in XALKORI?
Active ingredient: crizotinib
Capsules inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, and hard gelatin capsule shells.
Pink opaque capsule shell contains: gelatin, titanium dioxide, and red iron oxide.
White opaque capsule shell contains: gelatin and titanium dioxide.
Printing ink contains: shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Oral pellets inactive ingredients: The uncoated pellets contain poloxamer and stearyl alcohol. The film-coating contains hypromellose, glyceryl monostearate, medium chain triglycerides, polyethylene glycol/macrogol, sucrose, and talc.
LAB-0441-12.0
For more information, go to www.Pfizer.com or call 1-800-438-1985. -
Instructions for Use
INSTRUCTIONS FOR USE
XALKORI® [zal-KOR-ee]
(crizotinib)
oral pellets
This Instructions for Use contains information on how to give or take XALKORI oral pellets. Read this Instructions for Use each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider or pharmacist about your or your child’s medical condition or treatment.
Important information you need to know before giving or taking XALKORI oral pellets:
- •
- XALKORI oral pellets come in a capsule “shell” that must be opened before giving or taking a dose. Do not swallow the shell containing the oral pellets. Do not chew or crush the oral pellets.
- •
- XALKORI oral pellets come in 3 dosage strengths: 20 mg, 50 mg, and 150 mg. Your healthcare provider may combine different strengths for your prescribed dose. No more than 4 XALKORI oral pellet shells are to be used for a single dose.
- •
- Your healthcare provider will decide the right dose of XALKORI oral pellets for you or your child. Follow your healthcare provider’s instructions for the dose of XALKORI oral pellets to give your child or for you to take.
- •
- Empty XALKORI oral pellets from the shells as described in Steps 1 to 4 below.
- •
- Check the expiration date on the bottle containing XALKORI oral pellets. Do not use XALKORI oral pellets after the expiration date on the bottle has passed.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to prepare and give or take the prescribed dose of XALKORI oral pellets.
Supplies needed to give or take XALKORI oral pellets:
- •
- XALKORI oral pellet(s), as prescribed by your healthcare provider.
- •
- Spoon or medicine cup (optional). See Step 4 “Giving or taking XALKORI oral pellets”.
Preparing XALKORI oral pellets (Steps 1 to 3):
Giving or taking XALKORI oral pellets (Step 4): There are 2 options for giving or taking the oral pellets.
Storing XALKORI oral pellets:
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep XALKORI oral pellets and all medicines out of the reach of children.
Disposing of empty XALKORI oral pellet shells:
- •
- Dispose of (throw away) the empty XALKORI oral pellet shell(s) in the household trash.
- •
- Ask your pharmacist how to throw away medicines you no longer use or are expired.
For more information, go to www.Pfizer.com or call 1-800-438-1985.
LAB-1526-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 09/2023
- PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 20 mg Oral Pellets Label
- PRINCIPAL DISPLAY PANEL - 50 mg Oral Pellets Label
- PRINCIPAL DISPLAY PANEL - 150 mg Oral Pellets Label
-
INGREDIENTS AND APPEARANCE
XALKORI
crizotinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-8140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRIZOTINIB (UNII: 53AH36668S) (CRIZOTINIB - UNII:53AH36668S) CRIZOTINIB 250 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) Product Characteristics Color PINK (opaque) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code Pfizer;CRZ;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-8140-20 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/26/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202570 08/26/2011 XALKORI
crizotinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-8141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRIZOTINIB (UNII: 53AH36668S) (CRIZOTINIB - UNII:53AH36668S) CRIZOTINIB 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) Product Characteristics Color PINK (cap) , WHITE (body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code Pfizer;CRZ;200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-8141-20 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/26/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202570 08/26/2011 XALKORI
crizotinib capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-1500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRIZOTINIB (UNII: 53AH36668S) (CRIZOTINIB - UNII:53AH36668S) CRIZOTINIB 150 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLOXAMER 407 (UNII: TUF2IVW3M2) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE (light blue cap) , BLUE (light blue body) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Pfizer;CRZ;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-1500-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217581 11/20/2023 XALKORI
crizotinib capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0507 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRIZOTINIB (UNII: 53AH36668S) (CRIZOTINIB - UNII:53AH36668S) CRIZOTINIB 50 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLOXAMER 407 (UNII: TUF2IVW3M2) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color GRAY (cap) , GRAY (light gray body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code Pfizer;CRZ;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0507-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217581 11/20/2023 XALKORI
crizotinib capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0251 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CRIZOTINIB (UNII: 53AH36668S) (CRIZOTINIB - UNII:53AH36668S) CRIZOTINIB 20 mg Inactive Ingredients Ingredient Name Strength STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLOXAMER 407 (UNII: TUF2IVW3M2) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE (light blue cap) , WHITE (body) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code Pfizer;CRZ;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0251-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217581 11/20/2023 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Registrant - Pfizer Inc (113480771) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals 985052076 ANALYSIS(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251) , API MANUFACTURE(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251) Establishment Name Address ID/FEI Business Operations Pfizer Manufacturing Deutschland GmbH 341970073 ANALYSIS(0069-8140, 0069-8141) , MANUFACTURE(0069-8140, 0069-8141) , PACK(0069-8140, 0069-8141) , LABEL(0069-8140, 0069-8141) Establishment Name Address ID/FEI Business Operations Upjohn Manufacturing Ireland Unlimited Company 986030667 ANALYSIS(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251) , API MANUFACTURE(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251) Establishment Name Address ID/FEI Business Operations Pfizer Asia Manufacturing Pte Ltd 936889401 ANALYSIS(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251) , API MANUFACTURE(0069-8140, 0069-8141, 0069-1500, 0069-0507, 0069-0251)