Label: INFANTS IBUPROFEN- ibuprofen suspension
- NDC Code(s): 58602-227-04, 58602-227-07
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
-
Warnings
Allergy alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed.
Heart attack and stroke warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Sore throat warning
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
- Do not use
-
Ask a doctor before use if
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has problems or serious side effects from taking pain relievers or fever reducers
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child has high blood pressure, heart disease, liver cirrhosis, kidney disease, or had a stroke
- child has asthma
- child is taking a diuretic
- Ask a doctor or pharmacist before use if the child is
- When using this product
-
Stop use and ask a doctor if
- child experiences any of the following signs of stomach bleeding:
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- child has symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- the child does not get any relief within first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- child experiences any of the following signs of stomach bleeding:
- Keep out of reach of children.
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise use age.
- mL = milliliter
- measure with the dosing device provided. Do not use with any other device.
- dispense liquid slowly into the child's mouth, toward the inner cheek
- if needed, repeat dose every 6 to 8 hours
- do not use more than 4 times a day
Dosing Chart
Weight (lb)
Age (mos)
Dose (mL)
under 6 mos
ask a doctor
12 to 17 lbs
6 to 11 mos
1.25 mL
18 to 23 lbs
12 to 23 mos
1.875 mL
Other information
- store between 20° to 25°C (68° to 77°F)
- do not use if carton is opened or seal under cap is broken or missing
- Inactive ingredients
- Questions or comments?
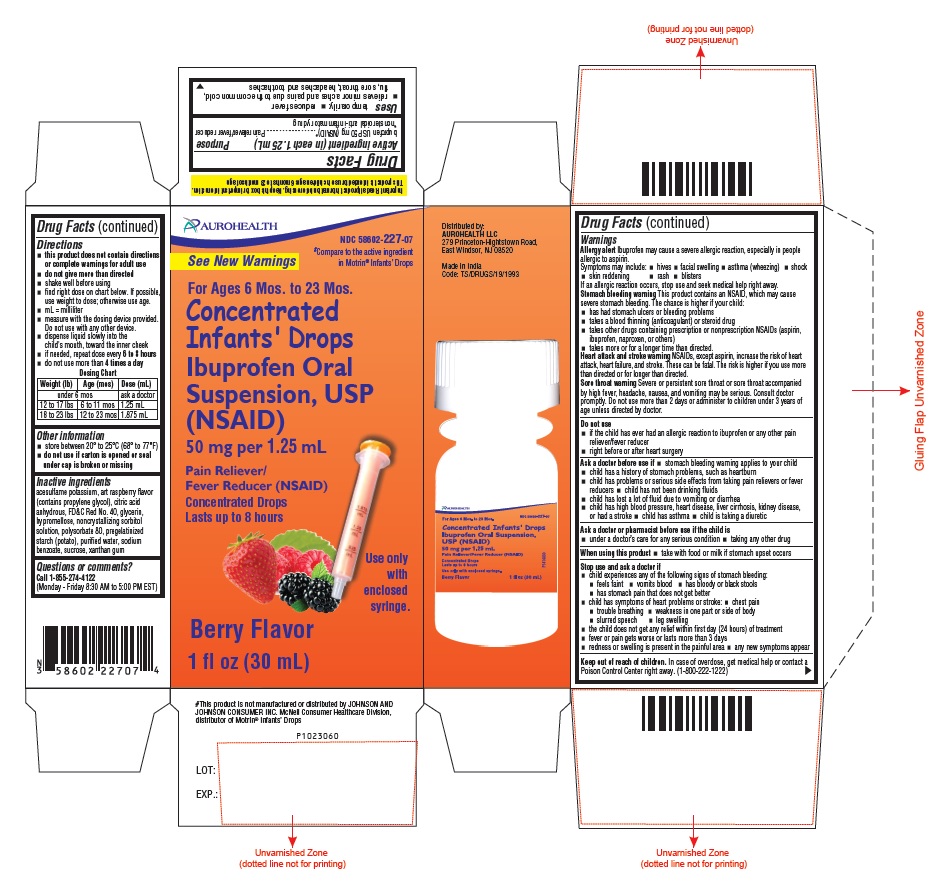
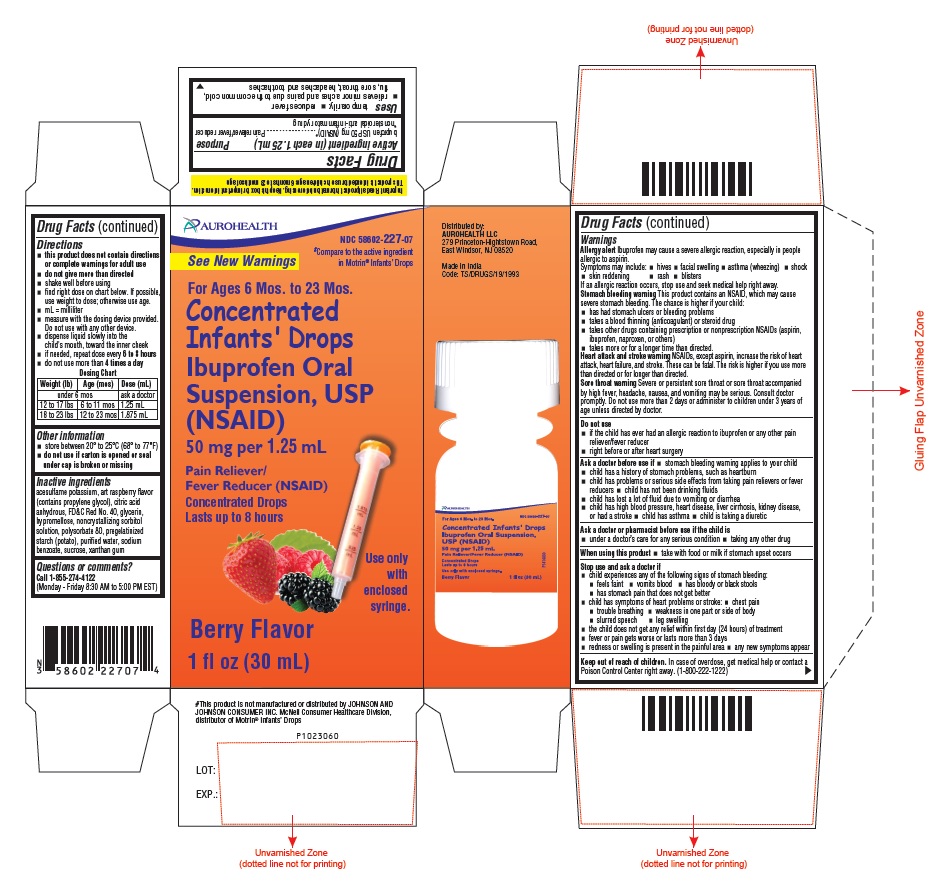
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 FL OZ (30 mL) Container Label
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 FL OZ (30 mL) Container Carton Label
AUROHEALTH
NDC 58602-227-07
#Compare to the active ingredient
in Motrin® Infants' Drops
See New Warnings
For Ages 6 Mos. to 23 Mos.
Concentrated
Infants' Drops
Ibuprofen Oral
Suspension, USP
(NSAID)
50 mg per 1.25 mL
Pain Reliever/
Fever Reducer (NSAID)
Concentrated Drops
Lasts up to 8 hours
Use only
with
enclosed
syringe.
Berry Flavor
1 fl oz (30 mL)
-
INGREDIENTS AND APPEARANCE
INFANTS IBUPROFEN
ibuprofen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-227 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 50 mg in 1.25 mL Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) NONCRYSTALLIZING SORBITOL SOLUTION (UNII: 9E0S3UM200) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, POTATO (UNII: 8I089SAH3T) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PINK (Light Pink to Pink) Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-227-04 1 in 1 CARTON 04/08/2024 1 15 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 2 NDC:58602-227-07 1 in 1 CARTON 04/08/2024 2 30 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213506 04/08/2024 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(58602-227) , MANUFACTURE(58602-227)