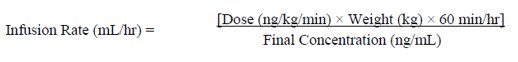Label: EPOPROSTENOL injection, powder, lyophilized, for solution
- NDC Code(s): 67457-587-10, 67457-588-10
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPOPROSTENOL FOR INJECTION safely and effectively. See full prescribing information for EPOPROSTENOL FOR INJECTION. EPOPROSTENOL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Epoprostenol for injection is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise capacity. Studies establishing effectiveness included ...
-
2 DOSAGE AND ADMINISTRATION Important Note:Reconstitute Epoprostenol for Injection only as directed with Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Do not dilute reconstituted solutions of ...
-
3 DOSAGE FORMS AND STRENGTHS Epoprostenol for Injectioncontains epoprostenol sodium equivalent to 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) epoprostenol and is supplied as a sterile lyophilized powder or cake in a 10 mL ...
-
4 CONTRAINDICATIONS A large study evaluating the effect of epoprostenol on survival in NYHA Class III and IV patients with congestive heart failure due to severe left ventricular systolic dysfunction was terminated ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Dose Initiation Epoprostenol is a potent pulmonary and systemic vasodilator. Initiate epoprostenol in a setting with adequate personnel and equipment for physiologic monitoring and ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS Additional reductions in blood pressure may occur when epoprostenol is administered with diuretics, antihypertensive agents, or other vasodilators. When other antiplatelet agents or anticoagulants ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Limited published data from case series and case reports with epoprostenol have not established a drug associated risk of major birth defects, miscarriage or ...
-
10 OVERDOSAGE Signs and symptoms of excessive doses of epoprostenol during clinical trials are the expected dose-limiting pharmacologic effects of epoprostenol, including flushing, headache, hypotension ...
-
11 DESCRIPTION Epoprostenol sodium is the sodium salt of epoprostenol, formulated as a sterile lyophilized powder for intravenous (IV) administration. Each vial of epoprostenol for injection contains ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Epoprostenol has 2 major pharmacological actions: (1) direct vasodilation of pulmonary and systemic arterial vascular beds, and (2) inhibition of platelet ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. A micronucleus test in rats revealed no ...
-
14 CLINICAL STUDIES 14.1 Clinical Trials in Pulmonary Arterial Hypertension (PAH) Acute Hemodynamic Effects - Acute intravenous infusions of epoprostenol for up to 15 minutes in patients with idiopathic or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Epoprostenol for injection is supplied as a sterile white to off-white lyophilized powder or cake in 10 mL vials. 10 mL vial with a white flip-off seal containing ...
-
17 PATIENT COUNSELING INFORMATION Patients receiving epoprostenol should receive the following information. Epoprostenol must be reconstituted as directed using only Sterile Water for Injection, USP, or Sodium Chloride 0.9 ...
-
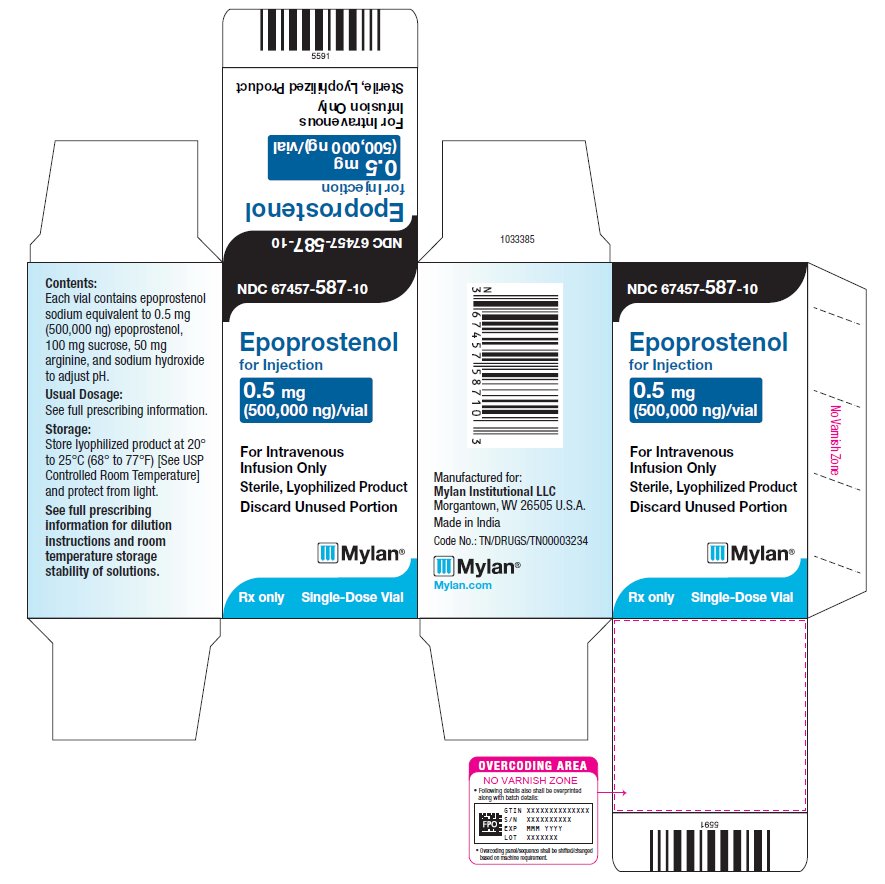
PRINCIPAL DISPLAY PANEL – 0.5 mg NDC 67457-587-10 - Epoprostenol - for Injection - 0.5 mg - (500,000 ng)/vial - For Intravenous - Infusion Only - Sterile, Lyophilized Product - Discard Unused Portion - Mylan® Rx only - Single-Dose ...
-
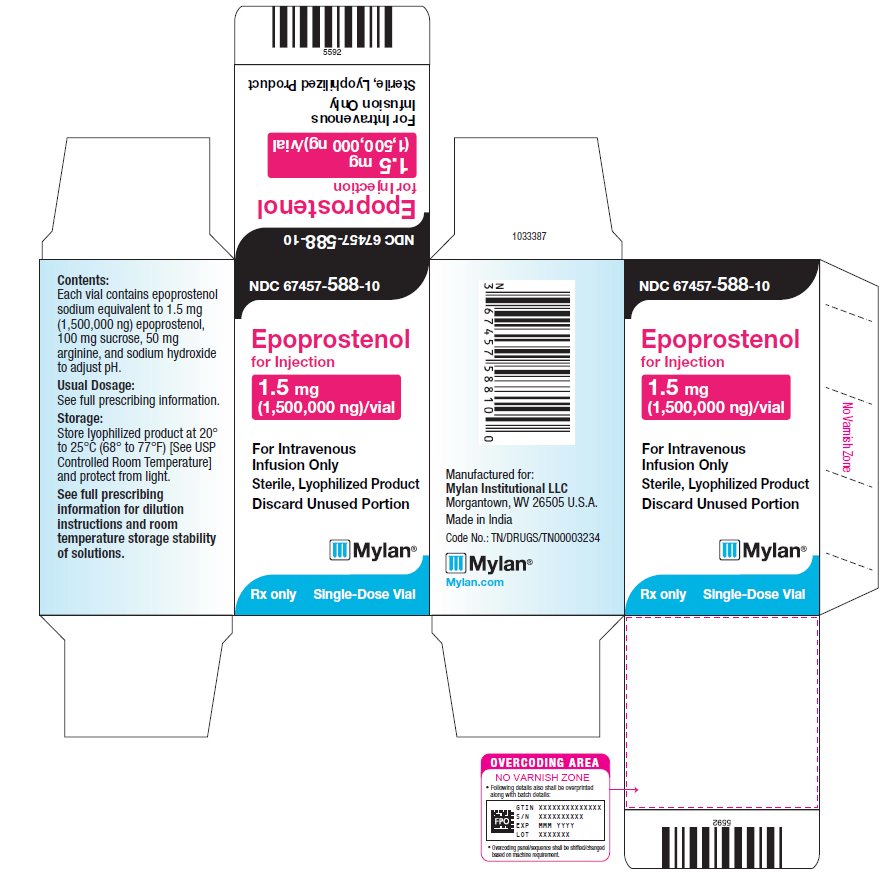
PRINCIPAL DISPLAY PANEL – 1.5 mg NDC 67457-588-10 - Epoprostenol - for Injection - 1.5 mg - (1,500,000 ng)/vial - For Intravenous - Infusion Only - Sterile, Lyophilized Product - Discard Unused Portion - Mylan® Rx only - Single-Dose ...
-
INGREDIENTS AND APPEARANCEProduct Information