Label: FLUTICASONE- fluticasone propionate spray, metered
- NDC Code(s): 68788-7788-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 60432-264
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FLUTICASONE PROPIONATE NASAL SPRAY safely and effectively. See full prescribing information for FLUTICASONE PROPIONATE NASAL SPRAY ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFluticasone Propionate Nasal Spray, USP 50 mcg per spray is indicated for the management of the nasal symptoms of perennial nonallergic rhinitis in adult and pediatric patients aged 4 years and ...
-
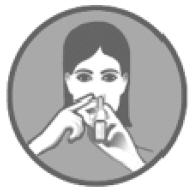
2 DOSAGE AND ADMINISTRATIONAdminister Fluticasone Propionate Nasal Spray by the intranasal route only. Prime Fluticasone Propionate Nasal Spray before using for the first time or after a period of non-use (1 week or more ...
-
3 DOSAGE FORMS AND STRENGTHSFluticasone Propionate Nasal Spray is an aqueous suspension. Each 100-mg spray delivers 50 mcg of fluticasone propionate.
-
4 CONTRAINDICATIONSFluticasone Propionate Nasal Spray is contraindicated in patients with hypersensitivity to any of its ingredients [see Warnings and Precautions (5.3), Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Local Nasal Effects - Epistaxis - In clinical trials of 2 to 26 weeks' duration, epistaxis was observed more frequently in subjects treated with Fluticasone Propionate Nasal Spray than those ...
-
6 ADVERSE REACTIONSSystemic and local corticosteroid use may result in the following: • Epistaxis, nasal ulceration, Candida albicans infection, nasal septal perforation, and impaired wound healing [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Inhibitors of Cytochrome P450 3A4 - Fluticasone propionate is a substrate of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data on the use of Fluticasone Propionate Nasal Spray in pregnant women to inform a drug-associated risk. In animals, teratogenicity ...
-
10 OVERDOSAGEChronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.5)]. Intranasal administration of 2 mg (10 times the recommended dose) of fluticasone propionate ...
-
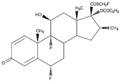
11 DESCRIPTIONThe active component of Fluticasone Propionate Nasal Spray is fluticasone propionate, a corticosteroid having the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate has been shown in vitro to exhibit a binding ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility - Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 20 times the ...
-
14 CLINICAL STUDIESPerennial Nonallergic Rhinitis: Three randomized, double-blind, parallel-group, vehicle placebo-controlled trials were conducted in 1,191 subjects to investigate regular use of Fluticasone ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFluticasone Propionate Nasal Spray, 50 mcg is supplied in an amber glass bottle fitted with a white metering atomizing pump, white nasal adapter, and transparent dust cap in a box of 1 (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Local Nasal Effects - Inform patients that treatment with Fluticasone Propionate ...
-
PATIENT PACKAGE INSERTPatient Information - Fluticasone Propionate Nasal Spray, USP - 50 mcg per spray - What is Fluticasone Propionate Nasal Spray? Fluticasone Propionate Nasal Spray is a prescription medicine used ...
-
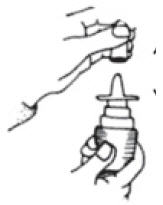
SPL UNCLASSIFIED SECTIONInstructions for Use - Fluticasone Propionate Nasal Spray - USP 50 mcg per spray - Fluticasone Propionate Nasal Spray is for use in your nose only. Read this information before you start using ...
-
PRINCIPAL DISPLAY PANEL Bottle BoxMGP - NDC 68788-7788-1 - FLUTICASONE - PROPIONATE - NASAL SPRAY, USP - 50 mcg per spray - For Intranasal Use Only - 120 Metered Sprays - (each spray contains 50 mcg - of fluticasone ...
-
INGREDIENTS AND APPEARANCEProduct Information