Label: PROPRANOLOL HYDROCHLORIDE tablet
- NDC Code(s): 43063-647-30, 43063-647-90
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0603-5484
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
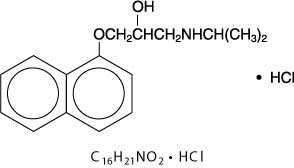
DESCRIPTIONPropranolol hydrochloride is a synthetic beta-adrenergic receptor blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,(±)-. Its ...
-
CLINICAL PHARMACOLOGYGeneral - Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor ...
-
PHARMACOKINETICS AND DRUG METABOLISMAbsorption - Propranolol is highly lipophilic and almost completely absorbed after oral administration. However, it undergoes high first-pass metabolism by the liver and on average, only about ...
-
PHARMACODYNAMICS AND CLINICAL EFFECTSHypertension - In a retrospective, uncontrolled study, 107 patients with diastolic blood pressure 110 to 150 mmHg received propranolol 120 mg t.i.d. for at least 6 months, in addition to ...
-
INDICATIONS AND USAGEHypertension - Propranolol hydrochloride tablets, USP are indicated in the management of hypertension. It may be used alone or used in combination with other antihypertensive agents, particularly ...
-
CONTRAINDICATIONSPropranolol is contraindicated in 1) cardiogenic shock; 2) sinus bradycardia and greater than first degree block; 3) bronchial asthma; and 4) in patients with known hypersensitivity to propranolol ...
-
WARNINGSAngina Pectoris - There have been reports of exacerbation of angina and, in some cases, myocardial infarction, following abrupt discontinuance of propranolol therapy. Therefore, when ...
-
PRECAUTIONSGeneral - Propranolol should be used with caution in patients with impaired hepatic or renal function. Propranolol is not indicated for the treatment of hypertensive emergencies. Beta-adrenergic ...
-
ADVERSE REACTIONSThe following adverse events were observed and have been reported in patients using propranolol. Cardiovascular:Bradycardia; congestive heart failure; intensification of AV block; hypotension ...
-
OVERDOSAGEPropranolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed: General: If ingestion is or may have been recent ...
-
DOSAGE AND ADMINISTRATIONGeneral - Because of the variable bioavailability of propranolol, the dose should be individualized based on response. Hypertension - The usual initial dosage is 40 mg propranolol hydrochloride ...
-
HOW SUPPLIEDPropranolol Hydrochloride Tablets, USP, 40 mg are green, round, convex, scored tablets, debossed “54” bisect “84” on one side and debossed “V” on the reverse side. They are available as ...
-
PRINCIPAL DISPLAY PANEL - 40 mg

-
INGREDIENTS AND APPEARANCEProduct Information