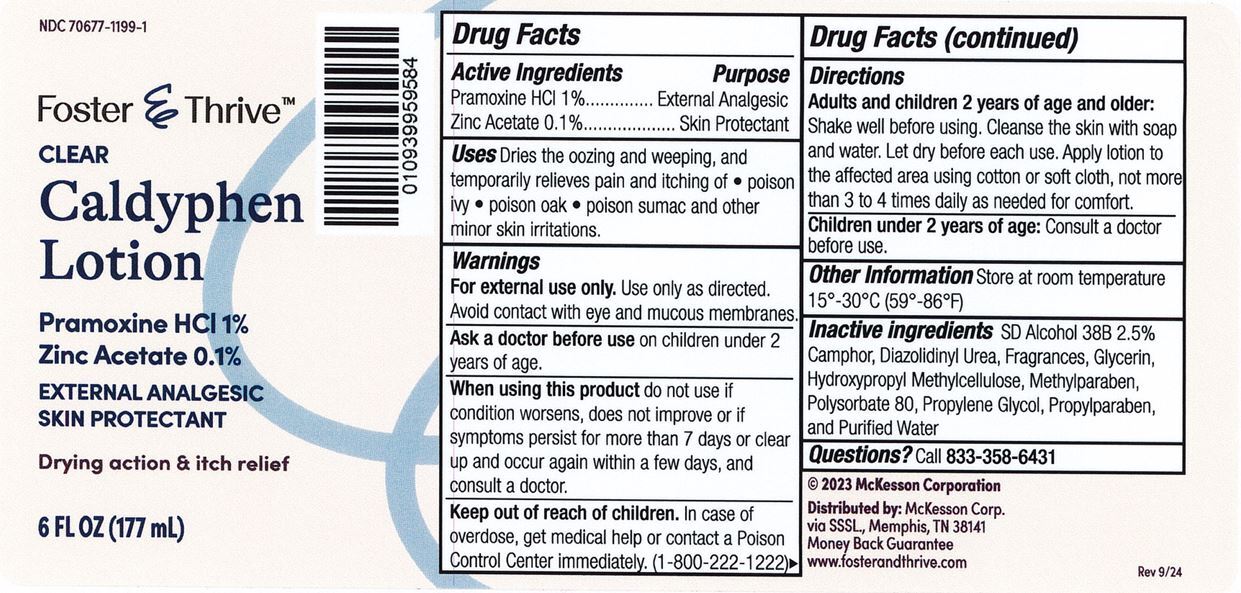
Label: FOSTER AND THRIVE CALDYPHEN CLEAR- pramoxine hydrochloride and zinc acetate lotion
- NDC Code(s): 70677-1199-1
- Packager: Strategic Sourcing Services, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Use
- Warnings
-
Directions
- Adults and children 2 yrs. of age and older. Shake well before using. Cleanse the skin with soap and water and let dry before each use. Apply lotion to the affected area using cotton or soft cloth, not more than 3 to 4 times daily as needed for comfort.
- Children under 2 years of age: Consult a doctor before use.
- Adults and children 2 yrs. of age and older. Shake well before using. Cleanse the skin with soap and water and let dry before each use. Apply lotion to the affected area using cotton or soft cloth, not more than 3 to 4 times daily as needed for comfort.
- Other Information
- Inactive Ingredient
- Questions?
- Label
-
INGREDIENTS AND APPEARANCE
FOSTER AND THRIVE CALDYPHEN CLEAR
pramoxine hydrochloride and zinc acetate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-1199 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 mL ZINC ACETATE (UNII: FM5526K07A) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-1199-1 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/18/2024 Labeler - Strategic Sourcing Services, LLC (116956644) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 analysis(70677-1199) , pack(70677-1199) , label(70677-1199) , manufacture(70677-1199)