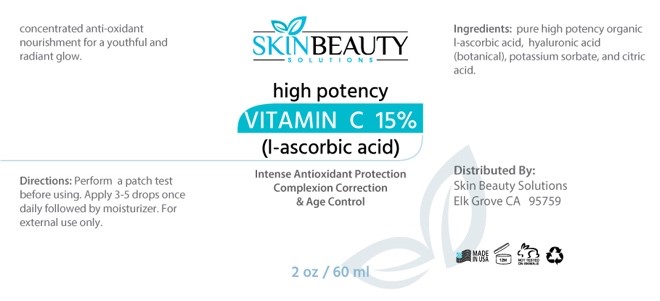
Label: VITAMIN C 15%- L- ASCORBIC ACID solution
- NDC Code(s): 84785-0053-1, 84785-0053-2
- Packager: Gazebo Wellness SKIN LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN C 15% (L- ASCORBIC ACID)
vitamin c 15% (l- ascorbic acid) solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84785-0053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 0.3 mg in 30 mL HYALURONIC ACID (UNII: S270N0TRQY) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONIC ACID 0.3 mg in 30 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID ACETATE (UNII: DSO12WL7AU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84785-0053-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2024 2 NDC:84785-0053-2 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/01/2024 Labeler - Gazebo Wellness SKIN LLC (119609953) Registrant - Gazebo Wellness SKIN LLC (119609953) Establishment Name Address ID/FEI Business Operations Gazebo Wellness SKIN LLC 119609953 manufacture(84785-0053)