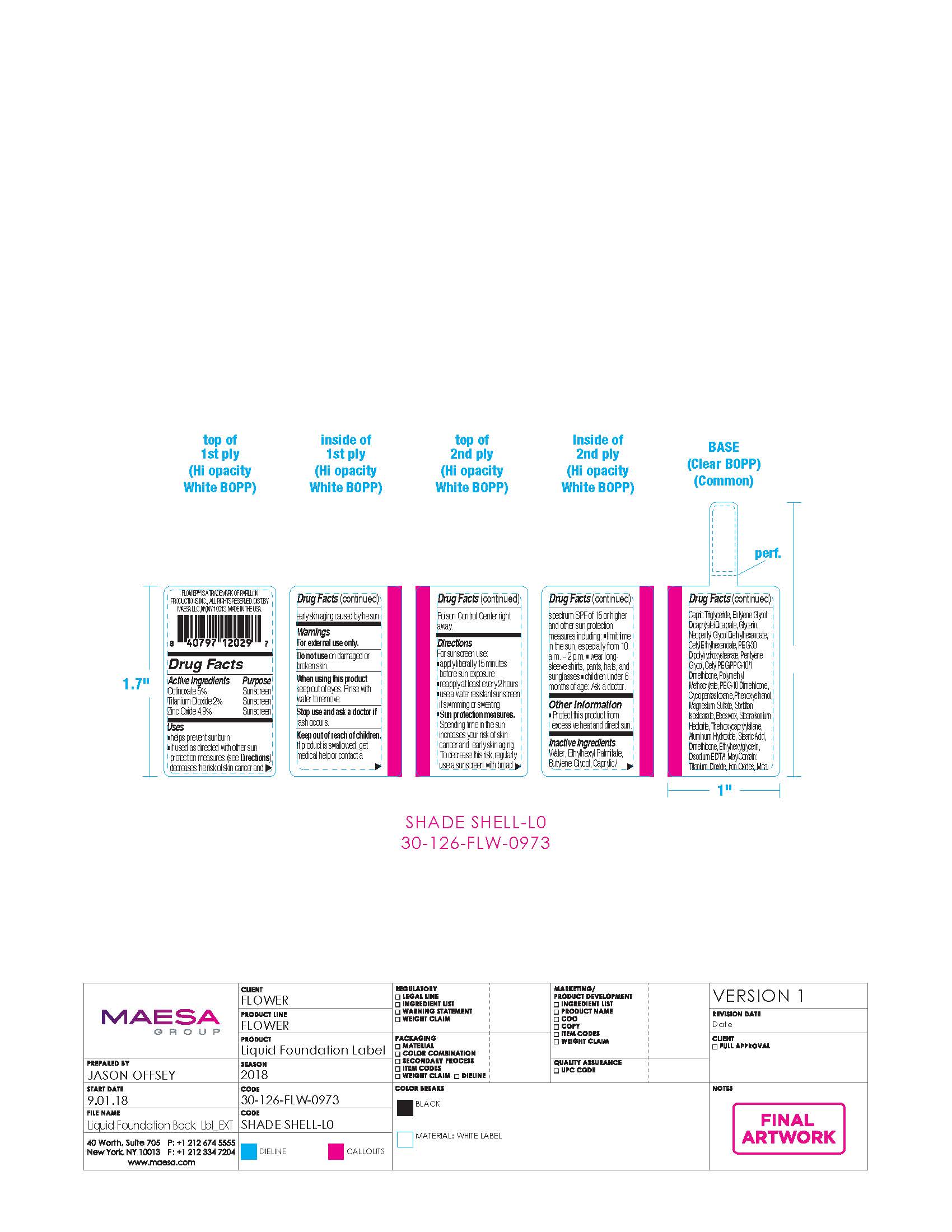
Label: LIGHT ILLUSION LUMINOUS MAKEUP NUDE-SKIN FEEL SPF18 SHELL- octinoxate, titanium dioxide, zinc oxide cream
- NDC Code(s): 68577-174-00
- Packager: COSMAX USA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
Drections
For sunscreen use:
- Apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: Water, Ethylhexyl Palmitate, Butylene Glycol, Caprylic/Capric Triglyceride, Butylene Glycol Dicaprylate/Dicaprate, Glycerin, Neopentyl Glycol Diethylhexanoate, Cetyl Ethylhexanoate, PEG-30 Dipolyhydroxystearate, Pentylene Glycol, Cetyl PEG/PPG-10/1 Dimethicone, Polymethyl Methacrylate, PEG-10 Dimethicone, Cyclopentasiloxane, Phenoxyethanol, Magnesium Sulfate, Sorbitan Isostearate, Beeswax, Stearalkonium Hectorite, Triethoxycaprylylsilane, Aluminum Hydroxide, Stearic Acid, Dimethicone, Ethylhexylglycerin, Disodium EDTA. May Contain: Titanium Dioxide, Iron Oxides, Mica.
- Packaging Label
-
INGREDIENTS AND APPEARANCE
LIGHT ILLUSION LUMINOUS MAKEUP NUDE-SKIN FEEL SPF18 SHELL
octinoxate, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-174 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.9 mg in 100 mg TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2 mg in 100 mg OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) CAPRYLIC/CAPRIC TRIGLYCERIDE (UNII: C9H2L21V7U) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) MAGNESIUM SULFATE (UNII: DE08037SAB) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BEESWAX (UNII: 2ZA36H0S2V) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTYLENE GLYCOL (UNII: 50C1307PZG) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) EDETATE DISODIUM (UNII: 7FLD91C86K) CYCLOPENTASILOXANE (UNII: 0THT5PCI0R) FERROUS OXIDE (UNII: G7036X8B5H) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-174-00 30 mg in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 02/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/15/2018 Labeler - COSMAX USA, INC (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA, CORPORATION 010990210 manufacture(68577-174)