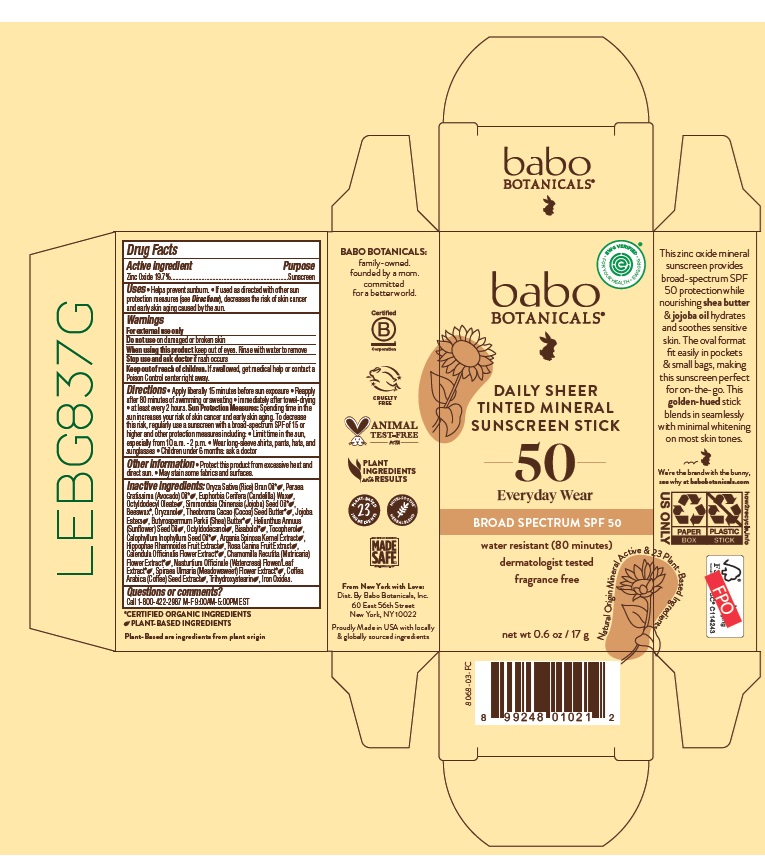
Label: BABO BOTANICALS DAILY SHEER TINTED MINERAL SUNSCREEN SPF 50- zinc oxide stick
- NDC Code(s): 79265-8608-1
- Packager: Babo Botanicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply after 80 minutes of swimming or sweating
- immediately after towel-drying
- at least every 2 hours.
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m,
- Wear long-sleeve shirts, pants, hats and sunglasses
- Children under 6 months: ask a doctor
- Other information
-
Inactive ingredients
Oryza Sativa (Rice) Bran Oil*, Persea Gratissima (Avocado) Oil*, Euphorbia Cerifera (Candelilla) Wax, Octyldodecyl Oleate, Simmondsia Chinensis (Jojoba) Seed Oil*, Beeswax*, Oryzanol, Theobroma Cacao (Cocoa) Seed Butter*, Jojoba Esters, Butyrospermum Parkii (Shea) Butter*, Helianthus Annuus (Sunflower) Seed Oil, Octyldodecanol, Bisabolol*, Tocopherol, Calophyllum Inophyllum Seed Oil*, Argania Spinosa Kernel Extract, Hippophae Rhamnoides Fruit Extract, Rosa Canina Fruit Extract, Calendula Officinalis Flower Extract*, Chamomilla Recutita (Matricaria) Flower Extract*, Nasturtium Officinale (Watercress) Flower/Leaf Extract*, Spiraea Ulmaria (Meadowsweet) Flower Extract*, Coffea Arabica (Coffee) Seed Extract, Trihydroxystearin, Iron Oxides.
*CERTIFIED ORGANIC INGREDIENTS
- Questions or comments?
- Product Labeling
-
INGREDIENTS AND APPEARANCE
BABO BOTANICALS DAILY SHEER TINTED MINERAL SUNSCREEN SPF 50
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79265-8608 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 197 mg in 1 g Inactive Ingredients Ingredient Name Strength FERROUS OXIDE (UNII: G7036X8B5H) FILIPENDULA ULMARIA FLOWER (UNII: 06L18L32G6) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) SUNFLOWER OIL (UNII: 3W1JG795YI) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) NASTURTIUM OFFICINALE FLOWERING TOP (UNII: W1N2U8I64G) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) JOJOBA OIL (UNII: 724GKU717M) COCOA BUTTER (UNII: 512OYT1CRR) ARGANIA SPINOSA SEED (UNII: 8H7X7XB54H) TOCOPHEROL (UNII: R0ZB2556P8) AVOCADO OIL (UNII: 6VNO72PFC1) RICE BRAN OIL (UNII: LZO6K1506A) CANDELILLA WAX (UNII: WL0328HX19) ARABICA COFFEE BEAN (UNII: 3SW678MX72) OCTYLDODECYL OLEATE (UNII: MCA43PK7MH) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) SHEA BUTTER (UNII: K49155WL9Y) CALOPHYLLUM TACAMAHACA SEED OIL (UNII: 05G85L61OE) CHAMOMILE (UNII: FGL3685T2X) BEESWAX (UNII: 2ZA36H0S2V) LEVOMENOL (UNII: 24WE03BX2T) OCTYLDODECANOL (UNII: 461N1O614Y) ORYZANOL (UNII: SST9XCL51M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79265-8608-1 1 in 1 CARTON 12/17/2024 1 17 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/17/2024 Labeler - Babo Botanicals, Inc. (058258734) Registrant - Bell International Laboratories (967781555)