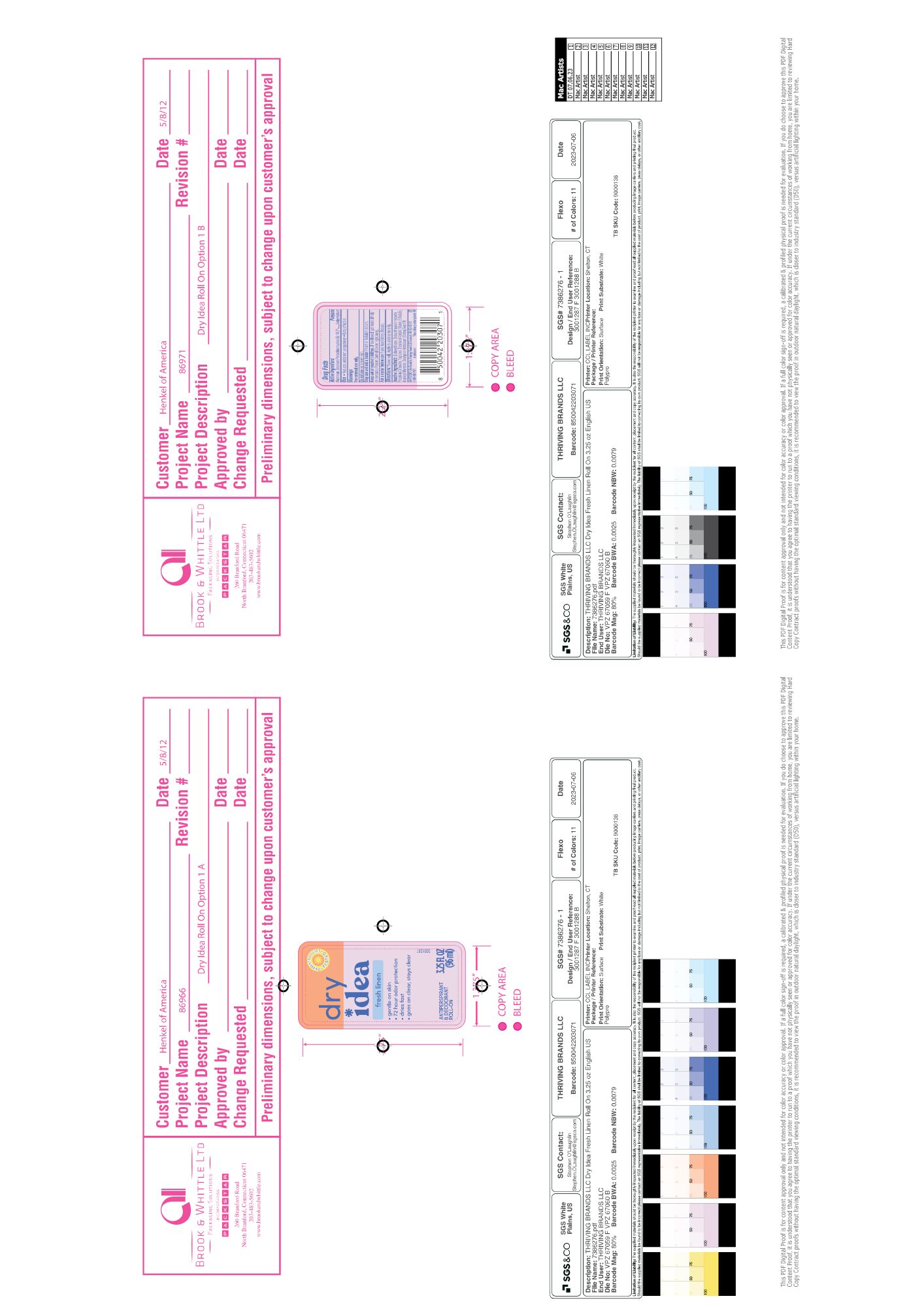
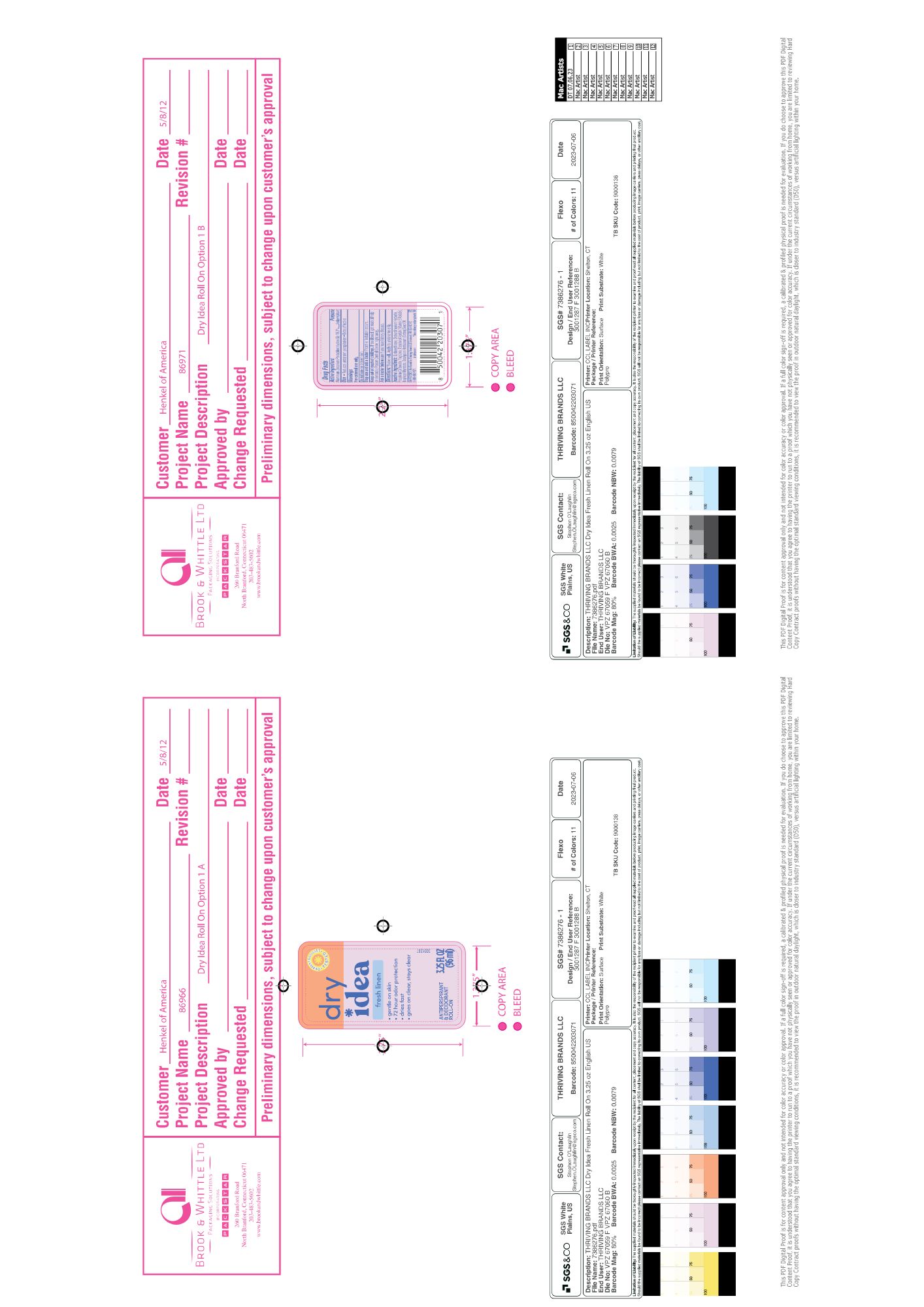
Label: DRY IDEA ADVANCED DRY ANTIPERSPIRANT ROLL-ON, FRESH LINEN liquid
- NDC Code(s): 82699-205-01
- Packager: Thriving Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- ASK DOCTOR
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRY IDEA ADVANCED DRY ANTIPERSPIRANT ROLL-ON, FRESH LINEN
dry idea advanced dry antiperspirant roll-on, fresh linen liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82699-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY (UNII: 94703016SM) (ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY - UNII:94703016SM) ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY 16.3 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE (UNII: NMQ347994Z) Product Characteristics Color brown (Light Brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82699-205-01 96 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 10/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 10/17/2024 Labeler - Thriving Brands LLC (118346160)