AUVI-Q® [Aw-Vee-Kyoo] (epinephrine injection)
Auto-Injector
-
For allergic emergencies (anaphylaxis)
Read this Patient Information Leaflet before you have to use AUVI-Q and each time ...
AUVI-Q® [Aw-Vee-Kyoo] (epinephrine injection)
Auto-Injector
For allergic emergencies (anaphylaxis)
Read this Patient Information Leaflet before you have to use AUVI-Q and each time you get a refill. There may be new information. You should know how to use AUVI-Q before you have an allergic emergency. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about AUVI-Q?
- Always carry AUVI-Q with you because you may not know when a life-threatening allergic reaction (anaphylactic reaction) may happen. Talk to your doctor if you need additional units to keep at work, school, etc. An anaphylactic reaction is a life-threatening allergic reaction that can happen within minutes and can be caused by stinging and biting insects (bees, wasps, hornets, and mosquitoes), allergy shots, foods, medicines, exercise, or other unknown causes. Follow your healthcare provider’s instructions on when to use AUVI-Q if you have the symptoms of an anaphylactic reaction, which may include the symptoms listed below:
- trouble breathing
- wheezing
- hoarseness (changes in the way your voice sounds)
- hives (raised reddened rash that may itch)
- severe itching
- swelling of your face, lips, mouth or tongue
- skin rash, redness, or swelling
- fast heartbeat
- weak pulse
- feeling very anxious
- confusion
- stomach pain
- losing control of urine or bowel movements
- dizziness or fainting
- Tell your family members and others where you keep AUVI-Q and how to use it before you need it. You may be unable to speak in an allergic emergency.
- Get medical attention immediately after using AUVI-Q. If you have a serious allergic reaction, you may need more medicine.
What is AUVI-Q?
AUVI-Q is a prescription medicine used to treat life-threatening allergic reactions including anaphylaxis in people who are at risk for or have a history of serious allergic reactions.
AUVI-Q is for immediate self (or caregiver) administration and does not take the place of emergency medical care. You should get emergency medical help right away after using AUVI-Q.
It is not known if AUVI-Q is safe and effective in children who weigh less than 16.5 pounds (7.5 kg).
What should I tell my healthcare provider before using AUVI-Q?
Before you use AUVI-Q, tell your healthcare provider if you:
- have heart problems or high blood pressure
- have diabetes
- have thyroid problems
- have history of depression
- have Parkinson’s disease
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if AUVI-Q will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AUVI-Q passes into your breast milk.
Tell your healthcare provider about all the medicines you take,
including prescription and non-prescription medicines, vitamins, and herbal supplements.
AUVI-Q and other medicines may affect each other, causing side effects. AUVI-Q may affect the way other medicines work, and other medicines may affect how AUVI-Q works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use AUVI-Q?
- Each AUVI-Q contains only 1 dose of medicine.
- AUVI-Q should only be injected into the muscle of your outer thigh. It can be injected through your clothing, if needed.
- Read the Instructions for Use at the end of this Patient Information Leaflet for information about the right way to use AUVI-Q.
- Use AUVI-Q exactly as your healthcare provider tells you to use it.
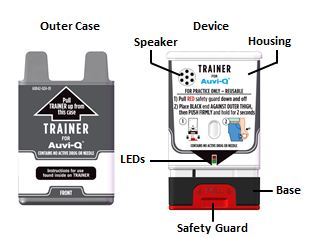
- You may request a Trainer for AUVI-Q. Additional training resources are available at
www.auvi-q.com.
- Practice with the Trainer for AUVI-Q before an allergic emergency happens to familiarize yourself with the use of AUVI-Q in an allergic emergency.
- The Trainer for AUVI-Q does not contain a needle or medicine and can be reused to practice your injection.
What are the possible side effects of AUVI-Q?
AUVI-Q may cause serious side effects.
-
AUVI-Q should only be injected into your outer thigh. Do notinject AUVI-Q into your:
- veins
- buttocks
- fingers, toes, hands or feet
If you accidentally inject AUVI-Q into any other part of your body, go to the nearest hospital emergency room right away. Tell the healthcare provider where on your body you received the accidental injection.
- Rarely, patients who use AUVI-Q may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
- If you inject a young child or infant with AUVI-Q, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to properly hold the leg of a young child or infant during an injection.
-
If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have more or longer lasting side effects when you use AUVI-Q. Talk to your healthcare provider about all your medical conditions.
Common side effects of AUVI-Q include:
- fast, irregular, or ‘pounding’ heart beat
- sweating
- shakiness
- headache
- paleness
- feelings of over excitement, nervousness, or anxiety
- weakness
- dizziness
- nausea and vomiting
- breathing problems
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of AUVI-Q. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store AUVI-Q?
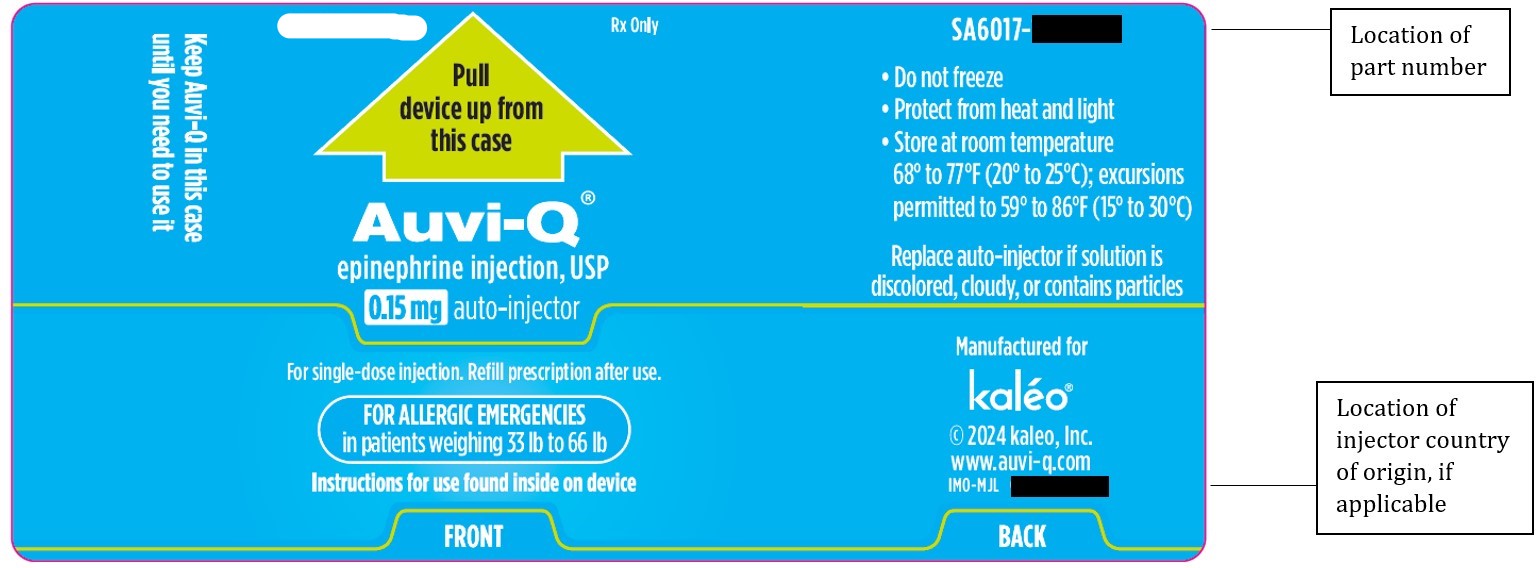
- Store AUVI-Q at 68° to 77°F (20° to 25°C).
- Do NOT freeze. Do NOT expose to extreme heat or cold. For example, do NOT store in your vehicle’s glove box.
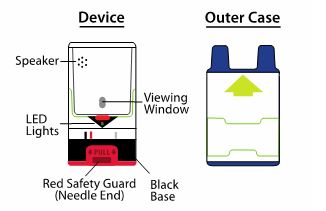
- Examine contents in the viewing window periodically. Solution should be clear. If the solution is discolored (pinkish color or darker than slightly yellow), cloudy or contains solid particles, replace the unit.
- Your AUVI-Q has an expiration date. Replace it before the expiration date.
- Keep AUVI-Q in the outer case it comes in to protect it from light.
Keep AUVI-Q and all medicines out of the reach of children.
General information about the safe and effective use of AUVI-Q:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use AUVI-Q for a condition for which it was not prescribed. Do not give AUVI-Q to other people, even if they have an allergic reaction or the same symptoms that you have. It may harm them.
This Patient Information Leaflet summarizes the most important information about AUVI-Q. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about AUVI-Q that is written for health professionals.
For more information and video instructions on the use of AUVI-Q, go to
www.auvi-q.comor call 1-877-302-8847.
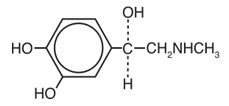
What are the ingredients in AUVI-Q?
Active ingredient:
epinephrine.
Inactive Ingredients:sodium chloride, sodium bisulfite, hydrochloric acid, and water.
AUVI-Q does not contain latex.
Instructions for Use
Read these Instructions for Use carefully before you need to use your AUVI-Q. Before you use AUVI-Q, make sure your healthcare provider shows you the right way to use it. If you have any questions, ask your healthcare provider.
If you are administering AUVI-Q to a young child or infant, hold the leg firmly in place and limit movement prior to and while administering an injection.
Automated Voice Instructions
AUVI-Q contains an electronic voice instruction system to help guide you through each step of your injection. If the voice instructions do not work for any reason, use AUVI-Q as instructed in these Instructions for Use. It will still work during an allergic reaction emergency.
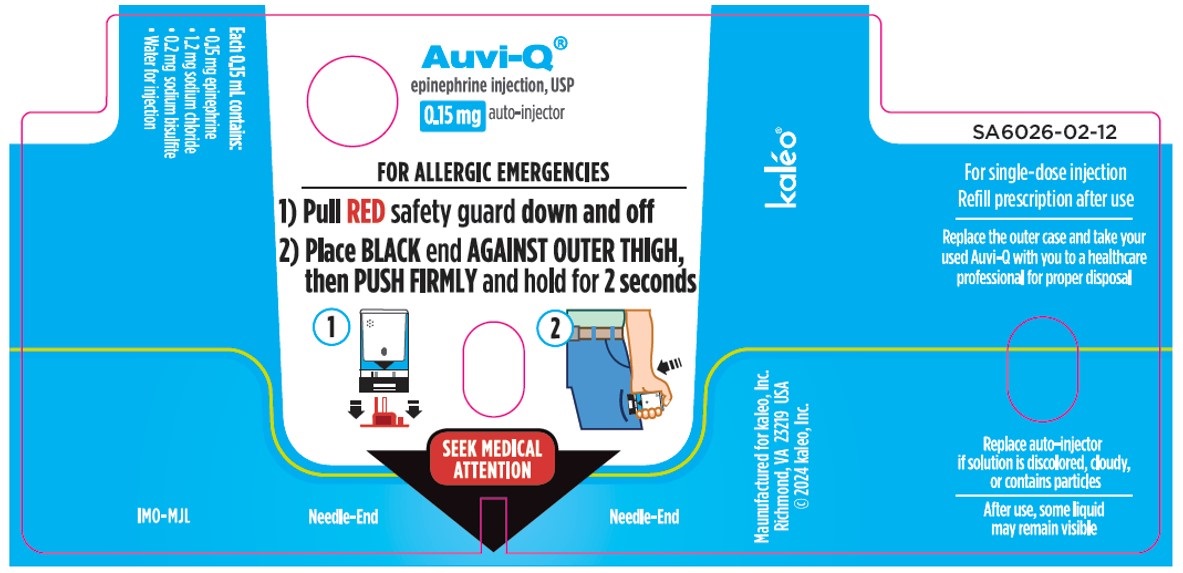
How to use your AUVI-Q
Figure A.
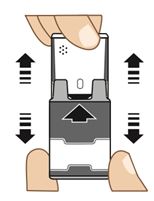
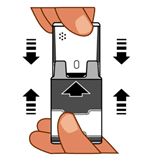
1. Pull AUVI-Q up from the outer case. See
Figure B.
Do not
go to step 2 until you are ready to use AUVI-Q. If you are not ready to use AUVI-Q, put it back in the outer case.
Figure B.
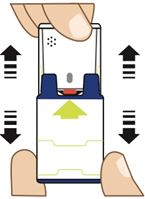
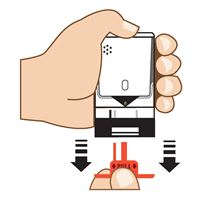
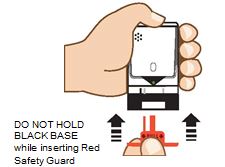
2. Pull Red safety guard down and off of AUVI-Q. See
Figure C.
To reduce the chance of an accidental injection, do not touch the black base of the auto-injector, which is where the needle comes out. If an accidental injection happens, get medical help right away.
Note:The red safety guard is made to fit tight.
Pull firmly to remove.
Figure C.
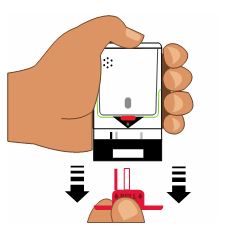
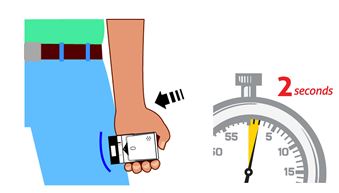
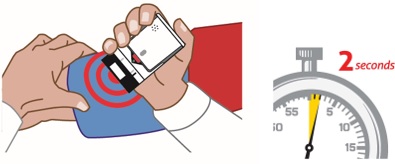
3. Place black end of AUVI-Q against the middle of the outer thigh (through clothing, if needed), then push firmly until you hear a click and hiss sound, and hold in place for 2 seconds. See
Figure D.
Onlyinject into the middle of the outer thigh.
Do notinject into any other part of the body.
If you are administering AUVI-Q to a young child or infant, hold the leg firmly in place while administering an injection See
Figure E.
Figure D.
(For AUVI-Q 0.3 mg and AUVI-Q 0.15 mg)
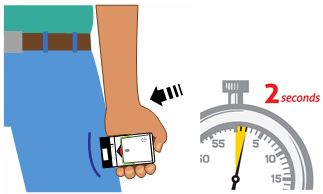
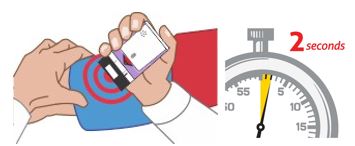
Figure E.
(For AUVI-Q 0.1 mg)
Note:AUVI-Q makes a distinct sound (click and hiss) when you push it against your outer thigh. This is normal and indicates AUVI-Q is working correctly. Do not pull AUVI-Q away from your leg when you hear the click and hiss sound.
The needle automatically retracts after the injection is complete, so the needle will not be visible after the injection. AUVI-Q includes a 2-second countdown after it is activated, then the voice instruction will indicate the injection is complete, and to seek emergency medical attention, AUVI-Q will beep, and the lights will blink red.
4. Get emergency medical help right away.
Replace the outer case and talk to your healthcare provider about the right way to throw away your AUVI-Q.
Ask your healthcare provider for an AUVI-Q prescription refill.
After the use of AUVI-Q:
- The black base will lock into place.
- The voice instruction system will say “seek emergency medical attention”, say “this AUVI-Q has been used…”, and the lights will blink red.
- Do not replace the red safety guard.
- The viewing window will no longer be clear.
- It is normal for some medicine to remain in your AUVI-Q after you have received your dose of medicine.
- Talk to your healthcare provider about the right way to throw away your AUVI-Q.
- AUVI-Q is a single-dose auto-injector and cannot be reused. AUVI-Q must be used or properly disposed once the red safety guard is removed.
Until you throw away your used AUVI-Q, the electronic voice instruction system will remind you that it has been used when the outer case is removed.
If you will be administering AUVI-Q to a young child or infant, ask your healthcare provider to show you how to properly hold the leg in place while administering a dose.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev. Feb 2024
Manufactured for:
kaleo, Inc.
Richmond, VA 23219 USA
This product may be covered by one or more U.S. patents or pending patent applications; see
www.kaleo.comfor details. KALÉO® and AUVI-Q® are registered trademarks of kaleo, Inc.
© 2024 kaleo, Inc.
*For California Only: This product uses batteries containing Perchlorate Material – special handling may apply. See
www.dtsc.ca.gov/hazardouswaste/perchlorate
Close