Label: ETHACRYNATE SODIUM injection, powder, lyophilized, for solution
- NDC Code(s): 70771-1106-1
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
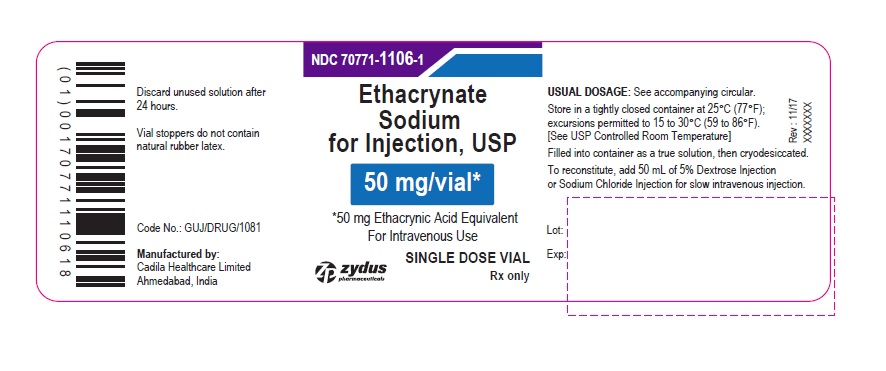
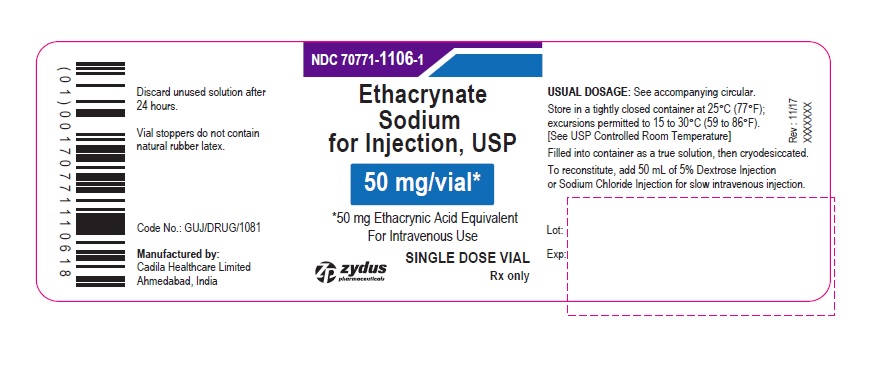
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 MG SINGLE DOSE VIAL CONTAINER LABEL
NDC 70771-1106-1
Ethacrynate Sodium for Injection, USP
50 mg/vial*
*50 mg Ethacrynic Acid Equivalent
For Intravenous Use
SINGLE DOSE VIAL
Rx only
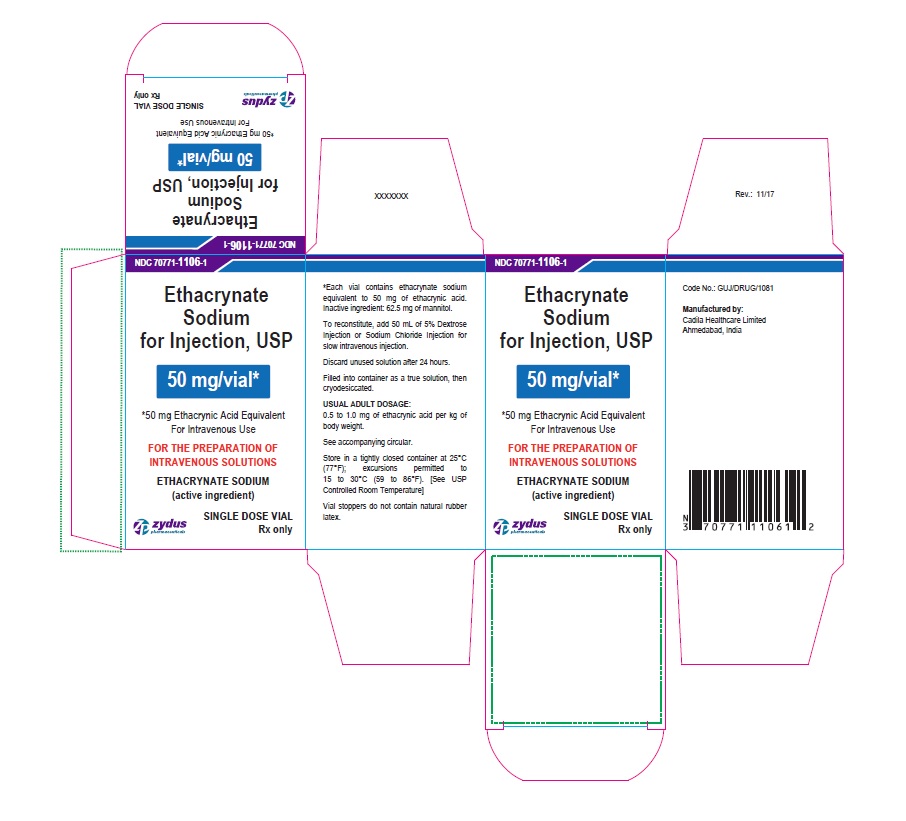
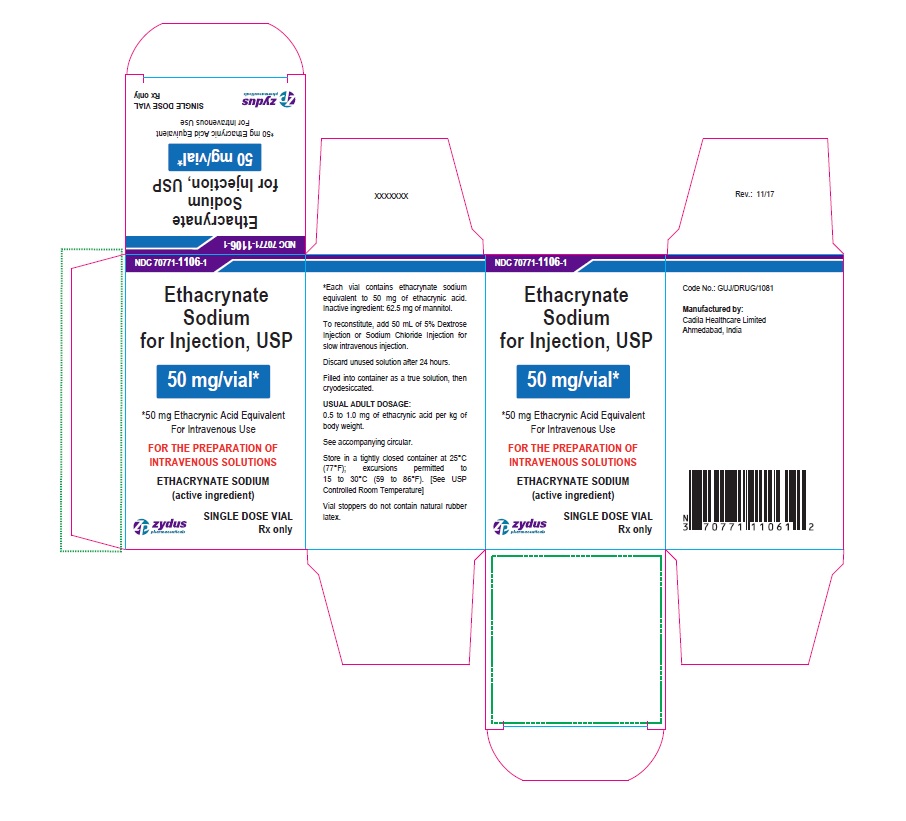
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 MG SINGLE DOSE VIAL CARTON LABEL
NDC 70771-1106-1
Ethacrynate Sodium for Injection, USP
50 mg/vial*
*50 mg Ethacrynic Acid Equivalent
For Intravenous Use
FOR THE PREPARATION OF INTRAVENOUS SOLUTIONS
ETHACRYNATE SODIUM (active ingredient)
SINGLE DOSE VIAL
Rx only
-
INGREDIENTS AND APPEARANCE
ETHACRYNATE SODIUM
ethacrynate sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1106 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHACRYNATE SODIUM (UNII: K41MYV7MPM) (ETHACRYNIC ACID - UNII:M5DP350VZV) ETHACRYNIC ACID 50 mg in 50 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 62.5 mg in 50 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1106-1 1 in 1 CARTON 01/24/2018 1 50 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207758 01/24/2018 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198)