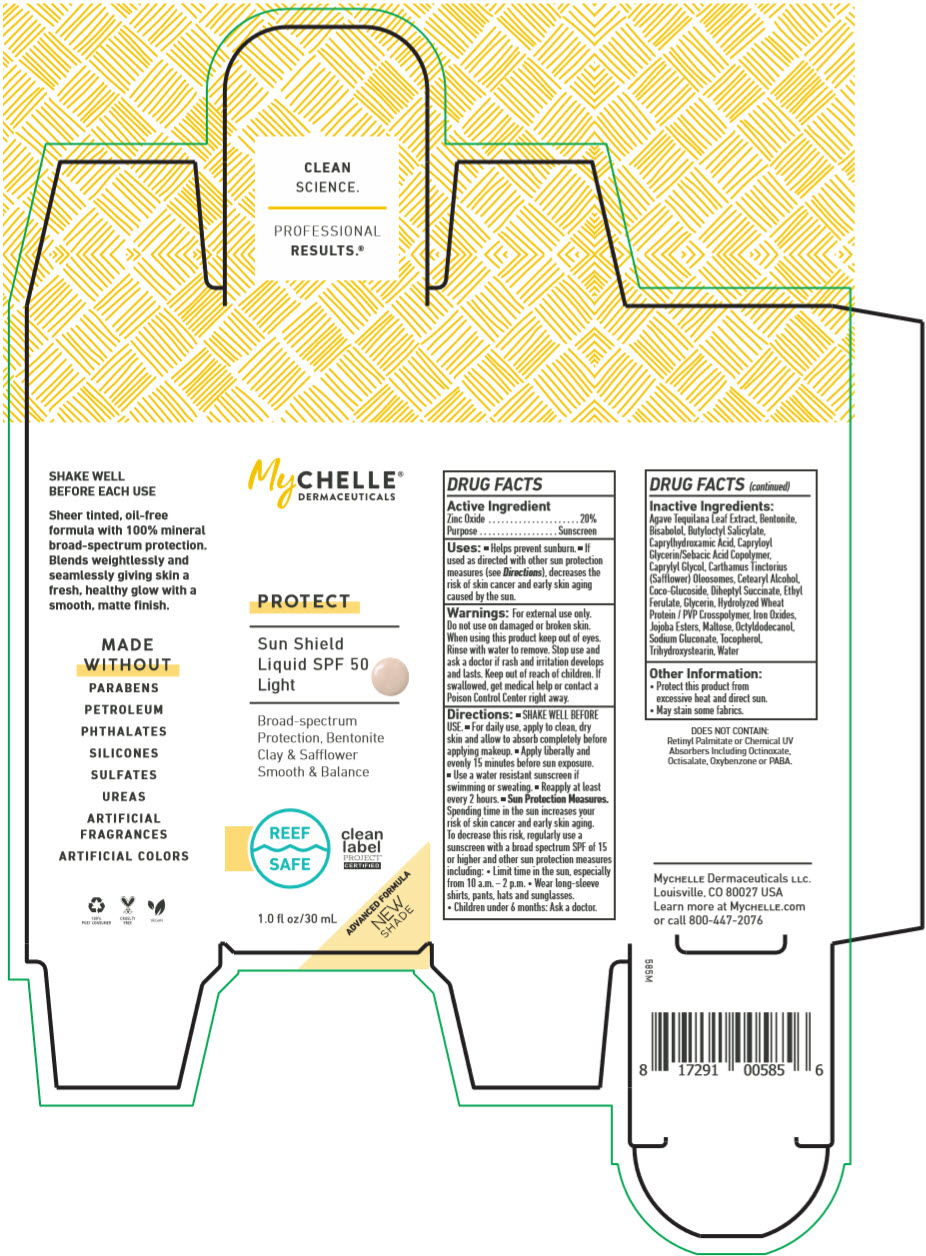
Label: MYCHELLE DERMACEUTICALS SUN SHIELD SPF 50 LIGHT PROTECT- zinc oxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 72805-180-30 - Packager: French Transit, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 24, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- SHAKE WELL BEFORE USE.
- For daily use, apply to clean, dry skin and allow to absorb completely before applying makeup.
- Apply liberally and evenly 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Agave Tequilana Leaf Extract, Bentonite, Bisabolol, Butyloctyl Salicylate, Caprylhydroxamic Acid, Capryloyl Glycerin/Sebacic Acid Copolymer, Caprylyl Glycol, Carthamus Tinctorius (Safflower) Oleosomes, Cetearyl Alcohol, Coco-Glucoside, Diheptyl Succinate, Ethyl Ferulate, Glycerin, Hydrolyzed Wheat Protein / PVP Crosspolymer, Iron Oxides, Jojoba Esters, Maltose, Octyldodecanol, Sodium Gluconate, Tocopherol, Trihydroxystearin, Water
- Other Information
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Box
-
INGREDIENTS AND APPEARANCE
MYCHELLE DERMACEUTICALS SUN SHIELD SPF 50 LIGHT PROTECT
zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72805-180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) AGAVE TEQUILANA LEAF (UNII: 05545M0E3M) Bentonite (UNII: A3N5ZCN45C) LEVOMENOL (UNII: 24WE03BX2T) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Caprylhydroxamic Acid (UNII: UPY805K99W) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) Caprylyl Glycol (UNII: 00YIU5438U) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCO GLUCOSIDE (UNII: ICS790225B) Diheptyl Succinate (UNII: 057N7SS26Y) Ethyl Ferulate (UNII: 5B8915UELW) Glycerin (UNII: PDC6A3C0OX) HYDROLYZED WHEAT PROTEIN (ENZYMATIC; 3000 MW) (UNII: J2S07SB0YL) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MALTOSE, UNSPECIFIED FORM (UNII: XJ6S9RV06F) Octyldodecanol (UNII: 461N1O614Y) Sodium Gluconate (UNII: R6Q3791S76) TOCOPHEROL (UNII: R0ZB2556P8) Trihydroxystearin (UNII: 06YD7896S3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72805-180-30 1 in 1 BOX 04/01/2021 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/01/2021 Labeler - French Transit, Ltd. (100044380)